Chromosomal Microarray Analysis (CMA) is a diagnostic test that can detect genetic imbalance in a fetus/ individual. Using anmiocytes/ CVS cells or blood, it is possible to quickly and thoroughly analyze all chromosomes in a single test. The sensitivity and specificity of CMA is much higher than the standard technique of karyotype analysis. Therefore, it is possible to identify clinically significant abnormalities that would previously undetected by karyotyping analysis.
Test target: This Fetal DNA Chip (version 2) is designed to extend the information to cover all common trisomic aneuploidies and also most of the known microdeletion and microduplication syndromes, including telomeric and peri-centromeric regions. Additionally, it enables to diagnose uniparental disomies and triploidies. This test is specifically designed to diagnose more than 100 recognized micro-deletion/duplication syndromes, and minimize the detection of variants of unknown clinical significance.
Note: Our previous web address http://www.fetalmedicine.hk/en/Fetal_DNA_chip.asp is no longer active and replaced by the current address http://www.obg.cuhk.edu.hk/_services/laboratory_service/chromosomal-microarray-analysis/
Test target: : Postnatal DNA Chip utilizes array-based comparative genomic hybridization (aCGH) with approximately 180,000 oligos covering the whole genome at the average resolution of 30Kb, 1,714 genes with all exons covered. The Postnatal DNA Chip can detect over 130 recognized genetic syndromes and other rare genetic disorders reported to be associated with mental retardation and/or physical problems. The CMA coverage can be search at https://www.bcm.edu/geneticlabs/disorder.cfm
Clinical information: Postnatal DNA Chip may be ordered for all patients with any indication of genomic imbalance which includes: dysmorphic features, unexplained mental retardation/developmental delay, autism spectrum disorder, and/or multiple congenital anomalies.
Turn Around Time (TAT): 1 month
Specimen requirement: Pretest genetic counseling is strongly recommended and a completed and signed consent form is required.
| Sample type | Amount |
| Blood | 2-3 ml in EDTA bottles |
| Saliva | Buccal swab |
| Purified DNA | At least 2ug minimal concentration of 50ng/uL; A260/A280 of ~1.8 |
Specimen submission:
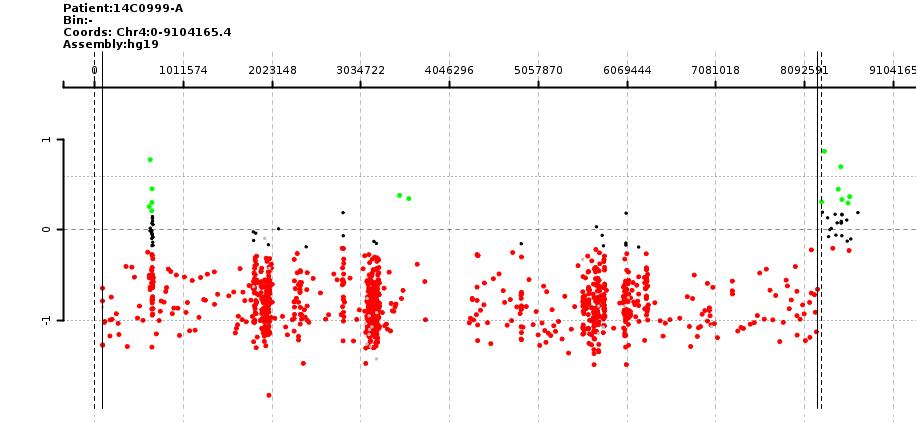
Fig. Postnatal DNA chip result of an individual consistent with Wolf-Hirschhorn syndrome (4p16.3-16.1 deletion).
