The Mystery of Molecular Matrilineality
August 2016
For 50 years scientists have known that DNA is not only found in the nucleus of a cell but also in the mitochondrion, often called the powerhouse of a cell because it generates most of the cell’s supply of chemical energy. But mitochondrial DNA (mDNA) only accounts for a small proportion of the total DNA in the human body (only about 37 genes out of a total of 20,000 are encoded from mDNA).
For 50 years scientists have puzzled over why in most animal species including humans, mDNA is inherited from the mother only, whilst nuclear DNA is contributed equally by both parents. Recently, Prof. Byung-Ho Kang, Associate Professor of CUHK’s School of Life Sciences and an expert in cell biology and advanced electron microscopy, has shed new light on why and how paternal mDNA is so conspicuously absent from a fertilized egg.
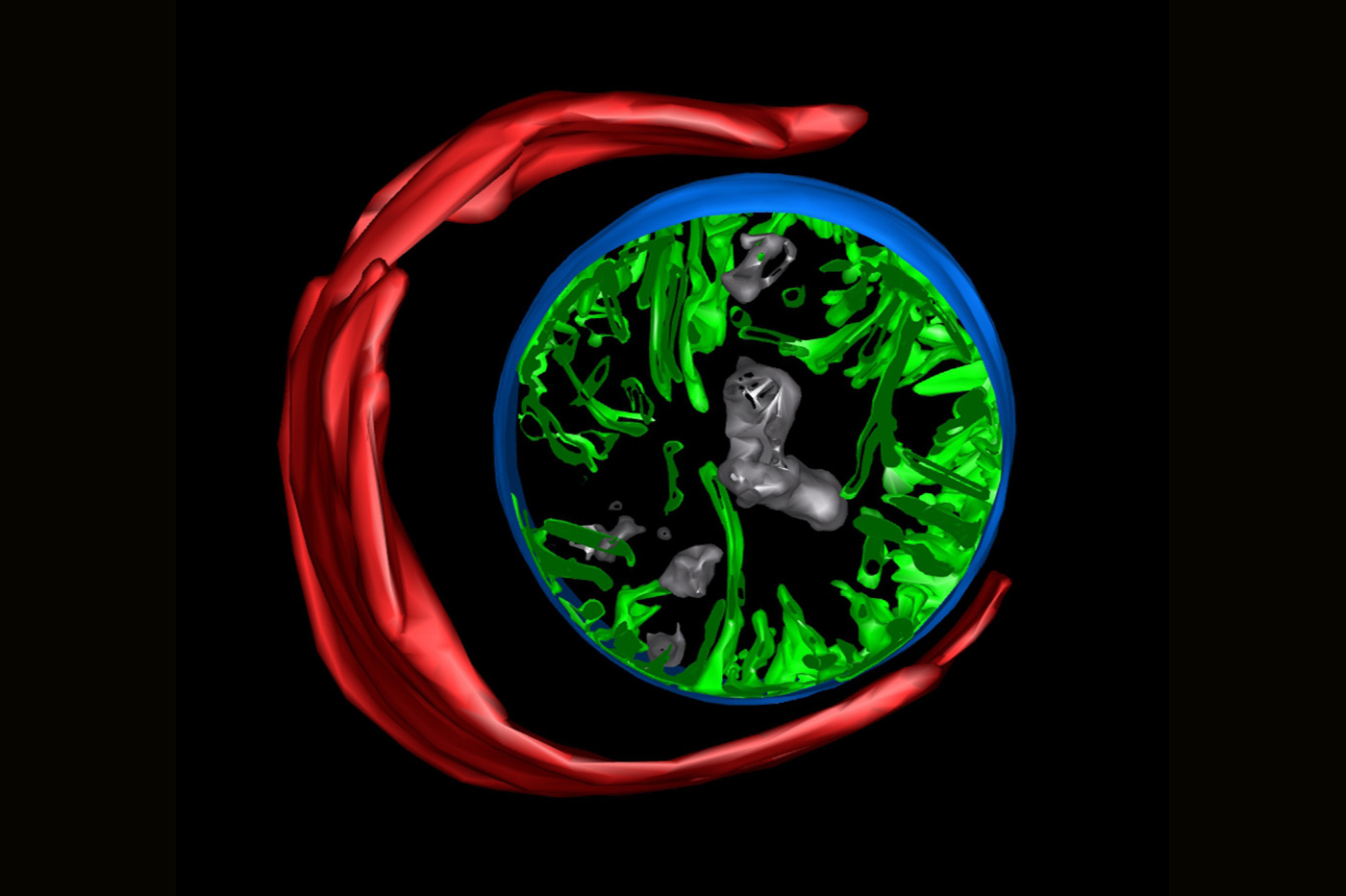
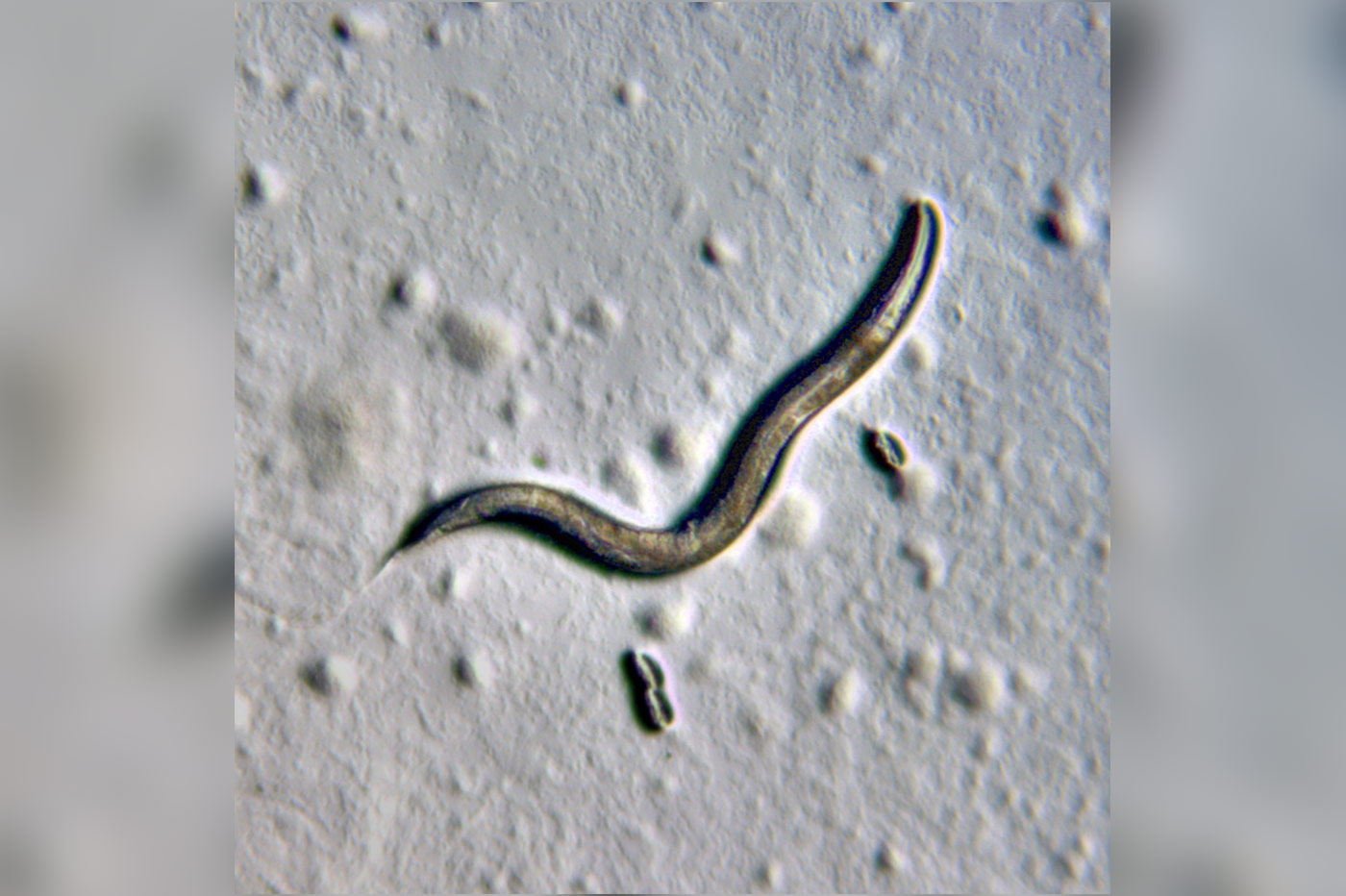
Professor Kang and Prof. Ding Xue’s group at the University of Colorado Boulder examined the mitochondria in the sperm of the roundworm Caenorhabditis elegans (C. elegans) using electron tomography that reveals cellular structures at nanometer-level resolutions in three-dimension. The 1-mm-long C. elegans which lives in temperate soil environments is the first multi-molecular organism to have its whole genome sequenced and is frequently used as a model organism for cellular research in biological organisms.
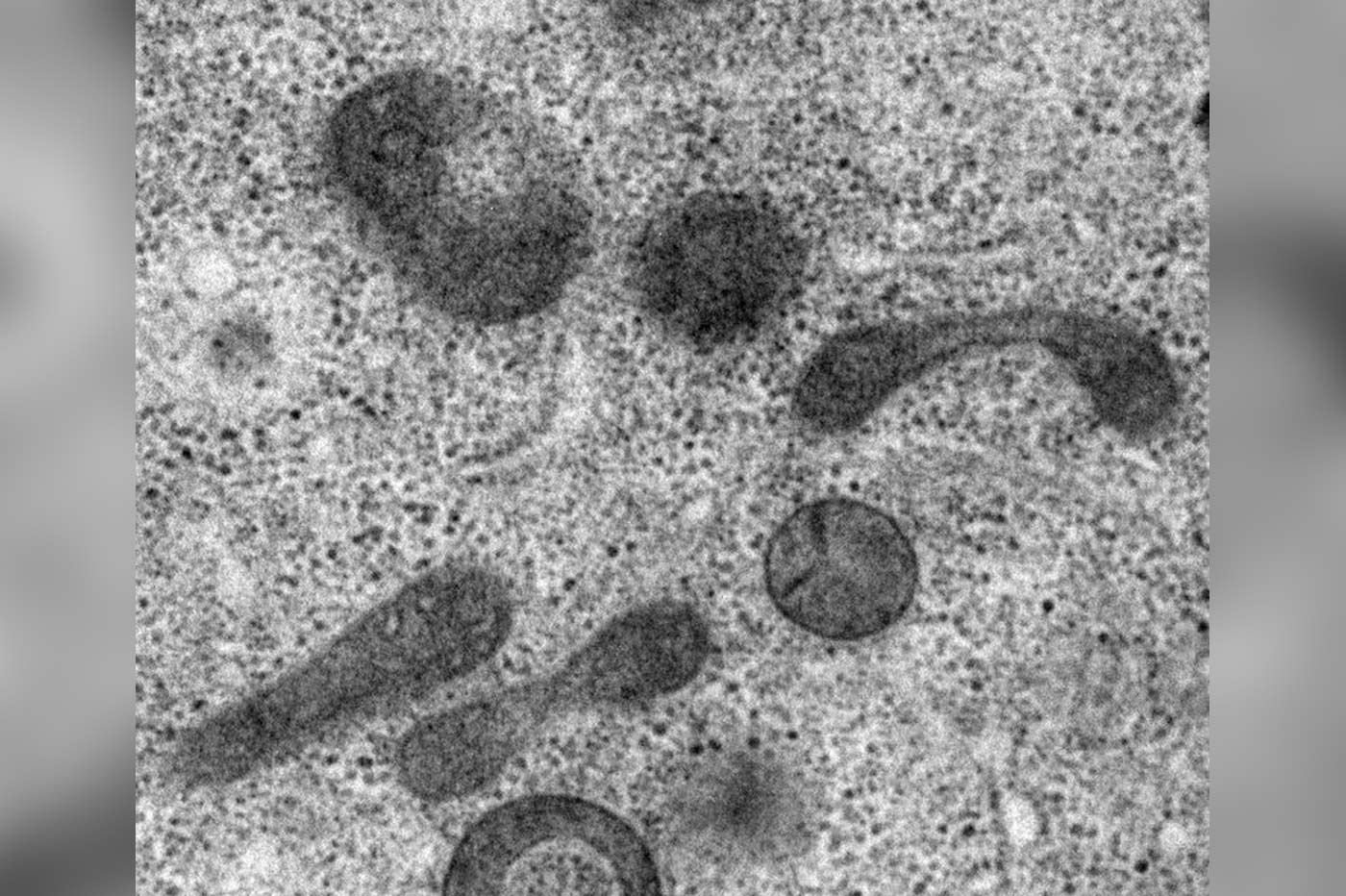
They observed that the sperm mitochondria in C. elegans exhibit signs of decay before the sperm reaches any autophagosome, a cell structure known to engulf sperm mitochondria in a process named autophagy (literally, ‘eating itself’) in the eggs of the organism.
Professor Kang said, ‘We found that the sperm mitochondria started their self-degradation as if they were committing suicide. This internal self-destruct mechanism gets activated once a sperm has penetrated an egg.’
The research team then searched for mitochondrial proteins that contribute to the self-initiated elimination of sperm mitochondria. They found that the protein CPS-6 localized on the surface of sperm mitochondria can chop off DNA molecules. These results provide a direct molecular explanation of how paternal mDNA is erased in the offspring.
They also found that paternal mitochondria could do harm in developing C. elegans embryos if they are not removed in time. Professor Kang said, ‘Delaying this mechanism will lower the survival rate of the embryo. This may help scientists better understand diseases related to this mechanism and improve in vitro fertilization techniques.’
Mitochondria are easily damaged in the process of producing energy for the cells. They are therefore under continuous surveillance of the autophagic system and any damaged or abnormal mitochondria will be removed. If this cleaning job is not done properly in humans, neurodegenerative diseases may develop. Further research on paternal mitochondrial elimination in C. elegans could provide new insights into autophagy in human cells and how to devise cures for diseases stemming from problems in autophagy, including Parkinson’s disease, dementia, some forms of heart diseases and blindness.
Professor Kang’s research findings have been published in Science. He remarked, ‘This research could not have been possible without the combined use of electron microscopic imaging and extensive searching for genes involved in the degradation of paternal mitochondria. Adopting a multi-disciplinary approach is critical for tackling difficult questions in biology. The success of this research exemplifies the importance of collaboration between laboratories with complementing expertise and techniques.’
Professor Kang is a member of the Centre for Organelle Biogenesis and Function, one of the four ongoing Areas of Excellence projects at CUHK funded and supported by the University Grants Committee of the HKSAR Government. He obtained his B. Sc. in Chemistry from Seoul National University and a Ph.D. in Biochemistry from the University of Wisconsin-Madison.
Professor Kang and his team are studying organelle biogenesis in animal and plant cells, combining the use of molecular biology and advanced electron microscopy techniques. His research with C. elegans has produced some very significant insights on apoptosis (programmed cell death) and autophagy.