© chemicalstructure.net
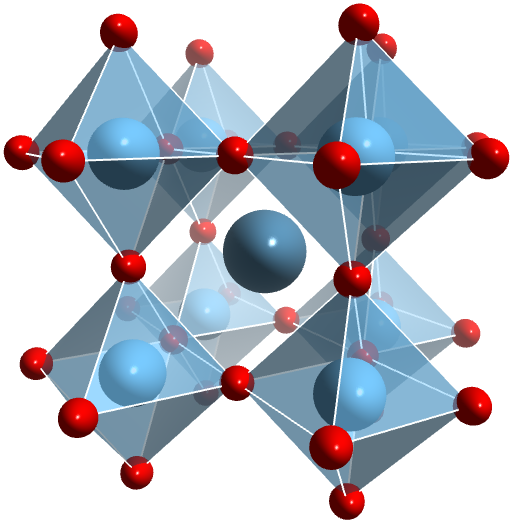
Organic-inorganic halide perovskite solar cells (PSCs) have attracted tremendous research attention due to the surge of its power conversion efficiency (PCE). Within 5 years, its PCE almost quintupled (>20%) since the first perovskite sensitized solar cell based on CH3NH3PbI3 and CH3NH3PbBr3 was demonstrated in 2009. Generally, perovskite names a class of material with a chemical formula of AMX3 and showing a similar crystal structure to CaTiO3. In our case, the organic and inorganic hybrid perovskite material for photovoltaic applications are typically organometal halides with A-site occupied by large organic cations, such as methylammonium (CH3NH3+) or formamidinium (HC(NH2)2+), M-site occupied by bivalent metal cations (e.g. Pb2+, Sn2+) and X-site by halide anions (e.g. Cl–, Br–, I–). Here, Octahedrons [MX6]4- form a three dimensional framework sharing corner X– ions with organic cation A+ filling in the voids. The overall structure of the framework is closely related to the size ratio of each component. Due to the combination of both organic and inorganic components, hybrid perovskites exhibit many properties that are superior for photovoltaic applications: wide absorption spectrum, tunable bandgap, weak exciton binding energy, high carrier mobility and low-temperature solution processability.