Overview
Overview
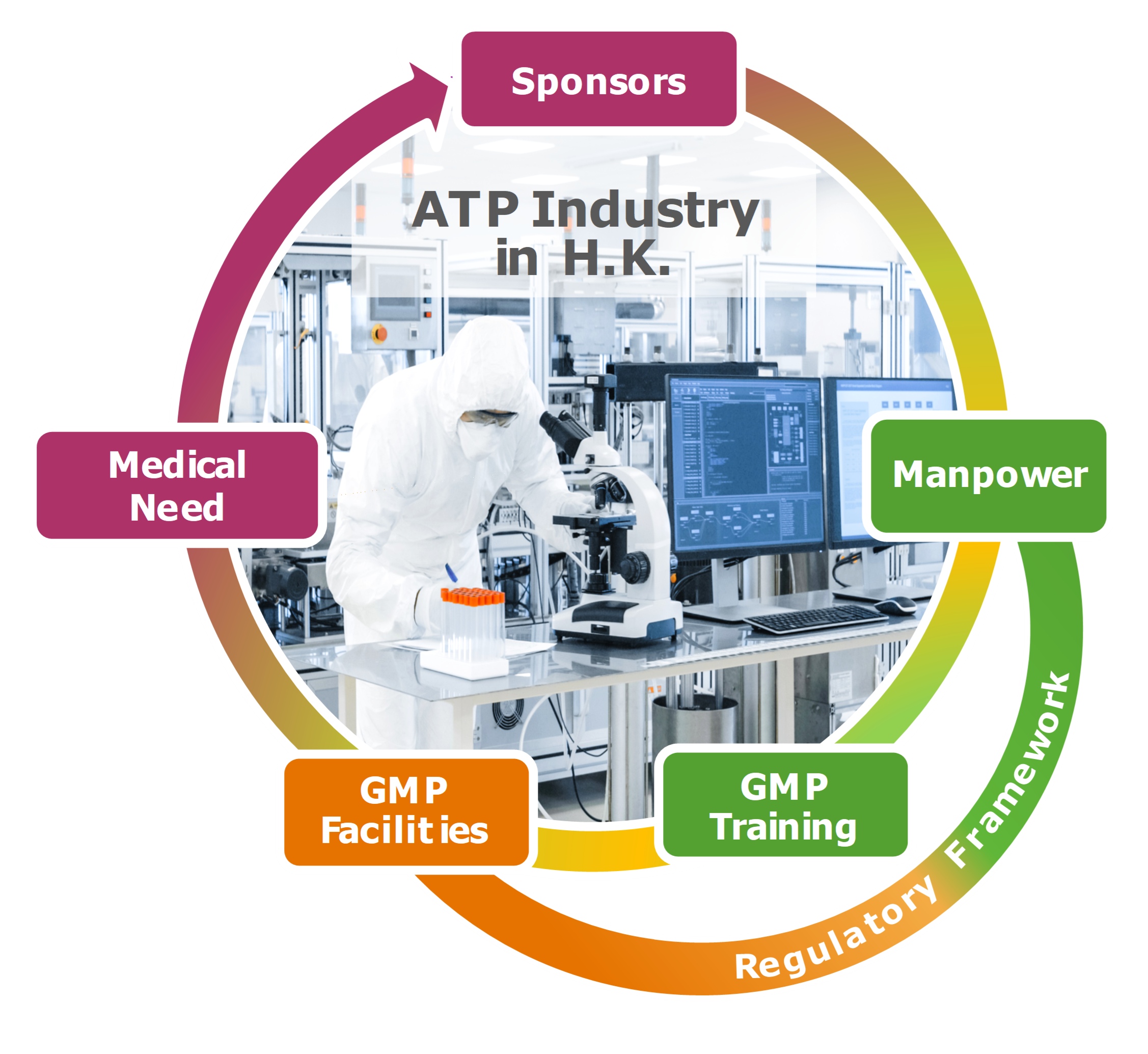
Advanced therapy products (ATP) are innovative medicinal products that encompass gene therapy, somatic cell therapy, and tissue-engineered products. The culmination of breathtaking advances in modern life sciences, ATP hold immense promise in meeting unmet medical needs in the treatment of genetic, hereditary, incurable, and rare diseases. As such, the regulatory authorities must be convinced that the principles of Current Good Manufacturing Practice (cGMP) are adhered to in the entire supply chain that underlies the manufacture of these innovative therapeutic products. The challenges include diverse country-specific requirements, new and orphan indications, novel technologies, inexperience of personnel, and complex manufacturing processes and facilities. The availability of manpower well versed in the science, technology, and manufacture of ATP is pivotal to satisfying the rising world-wide demand for ATP and to attracting investments to establish a new pharmaceutical industry in Hong Kong.
To assure the regulatory framework is in place to register cell therapy and other ATP, the Hong Kong SAR Government convened a task force in December 2017 to evaluate a proposal on registration, manufacturing, Quality Assurance (QA)/ Quality Control (QC), labeling, storage, documentation, and adverse event reporting requirements. The consultation document was open to the public for comments in April – June 2018. Of the many favorable comments received were requests of Good Manufacturing Practice (GMP) training of personnel and access to GMP facilities for academic laboratories, startups and clinical trial facilities. Importantly, on July 17, 2020, the Legislative Council of the Hong Kong SAR enacted the “ordinance to amend the Pharmacy and Poisons Ordinance and the Pharmacy and Poisons Regulation to regulate the manufacture, supply and labelling of advanced therapy products.”
The School of Pharmacy at the Chinese University of Hong Kong has developed a vigorous comprehensive training program comprising 5 short courses that can be taken individually or in combinations depending on the background of the applicant or the needs of the market. Colleagues at the School of Biomedical Sciences, the Drug Office of the Department of Health, and Hong Kong Science and Technology Parks Corporation were consulted throughout the planning of the program. The training program dovetailed the construction of an ATP GMP facility by CUHK very well. This facility will be the training site for students enrolled in the short course on Hands-on GMP training (PHAR5032).
Course Directors
 Course Director
Course Director
Prof. Joan Zuo
Director and Professor
School of Pharmacy
The Chinese University of Hong Kong
 Course Co-director
Course Co-director
Prof. Vincent Lee
Former Director
School of Pharmacy
The Chinese University of Hong Kong
5 Short Courses in Advanced Therapy Products
Students are encouraged to enroll in those courses which suit their needs. The School of Pharmacy offers 5 short courses to train students on the manufacture and regulation of the Advanced Therapy Products:
- Regulation of Advanced Therapy Products (PHAR5000)
- Scientific Rationale of Advanced Therapy Products (PHAR5010)
- Manufacture of Advanced Therapy Products (PHAR5020)
- Quality Control of Advanced Therapy Products (PHAR5030)
- Hands-on Good Manufacturing Practice Training (PHAR5032)
Program Structure and Proposed Study Scheme
| Offering Sequence | Ref. No. | Course Title | Teaching Hours | Proposed Offering Date |
| 1 | PHAR5000 | Regulation (產品規管) | 40 hrs | July 2021 |
| 2 | PHAR5010 | Scientific Rationale (科學原理) | 40 hrs Lecture Section & 20 hrs Laboratory Section (Pre-requisite of PHAR5032) * |
October 2021 |
| 3 | PHAR5020 | Manufacture (產品製造) | 40 hrs | January 2022 |
| 4 | PHAR5030 | Quality Control (質量控制) | 40 hrs | April 2022 |
| 5 | PHAR5032 * | Hands-on (實踐培訓) | 20 hrs | Coming soon |
| *Students who plan to take PHAR5032 Hands-on training must take PHAR5010 Scientific Rationale and its laboratory section and receive a passing grade. | ||||
Teaching Faculty
CUHK Teachers
School of Pharmacy
 Course Director
Course Director
Prof. Joan Zuo
Director and Professor
School of Pharmacy
The Chinese University of Hong Kong
Biographical Sketch
Biographical Sketch
Prof. Joan Zuo is the Director and Professor of the School of Pharmacy at the Chinese University of Hong Kong. She holds a B. Sc. and a Ph.D. in Pharmaceutical Sciences and has had over 20 years’ experience in the biopharmaceutics and pharmacokinetics fields. Since joining the School of Pharmacy at the Chinese University of Hong Kong in January 2000, Prof. Zuo has secured over 10 million continuous support from various granting agencies such as University Grant Council, Innovation Technology Foundation, Food and Health Bureau, Hospital Authority in Hong Kong SAR. Her research on the biopharmaceutics and quality of bioactive herbal components has created the framework for in vitro and in vivo models for evaluating the quality of complex herbal products, improvement in delivery of herbal components in vivo, elucidation or even prediction of potential herb/herb or herb/drug interactions in vivo. Prof. Zuo’s research findings in the above fields have generated over 300 original research and conference papers and patents of USA, China, Hong Kong and Malaysia.
In Hong Kong, Prof. Zuo is serving in the Pharmacy & Poisons Board, Pharmacy Internship Training Committee, Pharmacovigilance Committee, Traditional Chinese Medicine (TCM) Research and Development Committee, and Proprietary Traditional Chinese Medicine Registration Committee at the Government of Hong Kong Special Administration Region. She is currently the Council member of the Open University of Hong Kong and Expert Panel of the Hong Kong Science & Technology Parks Corporation.
Internationally, Prof. Zuo has served as nomination committee member for International Society of Xenobiotics (ISSX) and is currently the regional editor of European Journal of Pharmaceutical Sciences and editorial board member for Biopharmaceutics and Drug Dispositions, Xenobiotica, Chinese Medicine. She is also grant reviewer for National Natural Science Foundation of China (NSFC), Health and Medical Research Fund (HMRF) of Hong Kong, Macau Science and Technology Development Fund (FDCT), and as journal reviewer for more than 50 international peer reviewed journals.
Less
 Course Co-director
Course Co-director
Prof. Vincent Lee
Former Director
School of Pharmacy
The Chinese University of Hong Kong
Biographical Sketch
Biographical Sketch
Prof. Vincent Lee is the Former Director of School of Pharmacy Chinese University of Hong Kong (2006-2014). Prior to that he held senior management positions at the US Food and Drug Administration (FDA), ALZA (then a Johnson & Johnson company), and the University of Southern California. Prof. Lee is a world-renowned leader in controlled drug delivery. Through his leadership in scientific societies, scientific journals, and grant reviews, he was influential in setting the direction and quality of pharmaceutical research and drug development for decades. Prof. Lee joined the FDA at the time of radical transformation in the agency’s culture, when concepts such as Quality by Design (QbD) and Quality Risk Management (QRM) were championed and when a scientific approach to drug manufacturing and quality control was embraced. The present training program in pharmaceutical development and regulation of advanced therapy products is testament to Prof. Lee’s vision and leadership. Such a program is expected to play an indispensable role in building up the expert manpower necessary to establish and sustain a bourgeoning pharmaceutical industry in Hong Kong in the 21st century.
Less

Prof. Yin Ting Cheung
Assistant Professor
School of Pharmacy
The Chinese University of Hong Kong
Biographical Sketch
Biographical Sketch
Prof. Yin Ting Cheung received her training in pharmacoepidemiology and pharmacy practice from the National University of Singapore (NUS) and St. Jude Children’s Research Hospital (Memphis, USA). Her work with the Cognitive Neuroscience Team at St. Jude involved characterizing the pharmacological, biological and genetic determinants of brain function and other treatment-related complications in survivors of pediatric cancer. She had previous teaching appointments with Rhodes College (Memphis, USA) as an Adjunct Assistant Professor. She hopes that her collaborative research with students, clinicians and scientists can help to improve health outcomes in survivors of cancer and other chronic diseases.
Less

Prof. Sharon Leung
Assistant Professor
School of Pharmacy
The Chinese University of Hong Kong
Biographical Sketch
Biographical Sketch
Prof. Sharon Leung received her B. Eng. (Chemical Engineering) in 2007 from the Hong Kong University of Science and Technology and completed her PhD (Chemical Engineering) in the University of Sydney in 2012. After her PhD, she joined the Faculty of Pharmacy in the University of Sydney with interest in developing technology platforms for the production of engineering particles for respiratory delivery. In 2014, she was awarded a Postdoctoral Fellowship from the Faculty of Pharmacy to develop technologies for aerosol delivery of bacteriophages to combat multidrug-resistant (MDR) bacteria. Sharon joined the School of Pharmacy at the Chinese University of Hong Kong in April 2018. Her research focuses on incorporating engineering approach with formulation designs to advance the science and potential application of phage and phage-encoded proteins as novel antibacterial agents to address the threats associated with MDR bacteria.
Less

Prof. Billy Ng
Assistant Professor
School of Pharmacy
The Chinese University of Hong Kong
Biographical Sketch
Biographical Sketch
Prof. Billy Ng obtained his B.Sc. degree in Chemistry (1st Class Hons.) and Ph.D. in Organic Chemistry from the Chinese University of Hong Kong (CUHK). During his graduate study, he was a Fulbright Scholar at Massachusetts Institute of Technology (MIT), under the generous funding supports from the Lee Hysan Foundation and the Fulbright Program. From 2014 – 2016, he joined the University of Oxford as a Croucher Foundation Postdoctoral Fellow. He was then recruited to Harvard Medical School / Dana-Farber Cancer Institute as a research fellow from 2016 – 2019. His research interests are chemical biology, drug discovery, and medicinal chemistry. The Ng lab uses chemical, biological, and bioinformatics tools to develop novel small molecules for the treatment and diagnosis of various diseases, including cancers, diabetes, and neurodegenerative diseases.
Less
School of Biomedical Sciences

Prof. Hon Fai Vivas Chan
Assistant Professor
Institute for Tissue Engineering and
Regenerative Medicine
School of Biomedical Sciences
The Chinese University of Hong Kong
Biographical Sketch
Biographical Sketch
Prof. Hon Fai Vivas Chan is an Assistant Professor at the Institute for Tissue Engineering and Regenerative Medicine and School of Biomedical Sciences at The Chinese University of Hong Kong (CUHK). He received his B.Eng. in 2010 from The University of Hong Kong. He then pursued his M.S. and Ph.D. degree at Duke University with the support of the Sir Edward Youde Memorial Fellowships for Overseas Studies. During his Ph.D. training, he focused on developing several microfluidic technologies to perform 3D spheroid culture of mesenchymal stem cells and hepatocytes, and investigated the effect of supplementing extracellular matrix cues on spheroid functions. After graduation in 2015, Prof. Chan spent one year at Columbia University as a postdoctoral researcher. He performed high-throughput screening of synthetic genes for optimization of protein expression using microfluidic droplets. In 2016, Prof. Chan joined Massachusetts Institute of Technology as a postdoctoral associate. There he conducted organ-on-a-chip research and develop biomechanics-guided folded hydrogel to recapitulate morphogenesis of mucosal folding which is observed in many hollow or tubular organs in human body.
Less

Prof. Yangchao Chen
Professor
School of Biomedical Sciences
The Chinese University of Hong Kong
Biographical Sketch
Biographical Sketch
Prof. Yangchao Chen is currently a Professor at School of Biomedical Sciences, The Chinese University of Hong Kong (CUHK). He obtained his Ph.D. from Sun Yat-sen University in 2003 and later on was trained as a postdoctoral fellow at University of Washington, Seattle. He has been faculty member as Research Assistant Professor, Assistant Professor and Associate Professor at Faculty of Medicine CUHK since 2007. His research interests include epigenetics in cancer, histone modification particularly methylation, long and short non-coding RNAs, development of novel therapeutics for liver and pancreatic cancer. The ultimate goal of his lab is aimed at the identification of novel diagnostic markers and therapeutic targets for pancreatic and liver cancer.
Less

Prof. Bo Feng
Associate Professor
School of Biomedical Sciences
The Chinese University of Hong Kong
Biographical Sketch
Biographical Sketch
Prof. Bo Feng is an Associate Professor in the School Biomedical Sciences, Faculty of Medicine, The Chinese University of Hong Kong (CUHK). She is an active staff member in the Developmental and Regenerative Biology Thematic Research Program, Institute for Tissue Engineering and Regenerative Medicine, Ministry of Education (MoE) Key Laboratory for Regenerative Medicine and CUHK- Guangzhou Institute of Biomedicine and Health, Chinese Academy of Sciences (GIBH) Joint Laboratory on Stem Cell and Regenerative Medicine. Prof. Feng graduated from Nankai University with B.Sc. (1993) and M.Sc (1996), and received her Ph.D. (2006) from National University of Singapore. After graduation, Prof. Feng joined Prof. Ng Huck Hui’s lab in Genome Institute of Singapore as a postdoc. She worked on stem cells and reprogramming and published her works in Nature Cell Biology, Cell Stem Cell and Nature. In Nov 2010, Prof. Feng joined CUHK and her current research interest lies within the molecular mechanism that controls pluripotency and differentiation of Embryonic stem cells (ESCs)/ Induced pluripotent stem cells (iPSCs), as well as development of new tools for gene and cell-based therapy.
Less

Prof. Xiaohua Jiang
Associate Professor
School of Biomedical Sciences
The Chinese University of Hong Kong
Biographical Sketch
Biographical Sketch
Prof. Jiang is graduated from Shanghai Second Medical University (currently School of Medicine, Shanghai JiaoTong University) and completed her internship and residency at RuiJin Hospital, Shanghai. She obtained her PhD degree in cell biology from the University of Hong Kong. Prof. JIANG undertook her postdoctoral training at the Department of Medicine, UCLA. Her work focused on the role of protein kinase cascades in cancer development. After that, she joined the University of Southern California as a California Institute for Regenerative Medicine (CIRM) fellow and her research focused on understanding the origin and genetics of Ewing sarcoma by using human embryonic stem cells as an innovative model. In 2013, she established an independent laboratory within School of Biomedical Sciences at The Chinese University of Hong Kong. Her major research interest is in stem cell biology and regenerative medicine, in particular, molecular regulation of stem cells, stem cell microenvironment and stem cell therapy in neurological diseases. Prof. JIANG has published more than 80 peer-reviewed papers with over 3,000 citations and a h-index of 30, including Nature Medicine, Cell Research, Cell Death and Differentiation, Stem Cells, Stem Cell Reports and Cancer Research.
Less

Prof. Elmer Ker
Assistant Professor
Institute for Tissue Engineering and
Regenerative Medicine
School of Biomedical Sciences
The Chinese University of Hong Kong
Biographical Sketch
Biographical Sketch
Prof. Elmer Ker completed his Ph.D. in Biological Sciences from Carnegie Mellon University and postdoctoral training from the Department of Orthopaedic Surgery at Stanford University. He is an Assistant Professor at The Chinese University of Hong Kong with appointments in the School of Biomedical Sciences and the Institute for Tissue Engineering and Regenerative Medicine. His research interests include developing biomaterials and computer vision-based approaches for repairing injured bone-tendon and bone-ligament tissue units.
Less

Dr. Ann Lau
Principal Lecturer
School of Biomedical Sciences
The Chinese University of Hong Kong
Biographical Sketch
Biographical Sketch
Dr. Ann Sin Nga Lau is a Principal Lecturer in the School of Biomedical Sciences, The Chinese University of Hong Kong and is the Programme Director of BSc in Biomedical Sciences. She received her BSc (Biology, first class Honours) and PhD (Molecular Endocrinology) from The University of Hong Kong, with her doctoral training at Population Council, Centre for Biomedical Research (CBR) in Rockefeller University, New York. With her research interest in molecular endocrinology, she studies the roles of trafficking molecules at the epithelial tight and adherens junctions, and the mechanisms of non-hormonal approach to disrupt Sertoli-germ cell interactions. She is experienced in curriculum development and had developed a wide range of courses including Faculty Package, undergraduate courses for Biomedical Sciences, Pharmacy, and Biomedical Engineering Programmes. She is interested in teaching topics ranged from human genetics, endocrinology, reproductive biology, bioethics, biotechnology, to specialized topics like disorders of sex development (DSDs). She is the recipient of the Faculty’s Education Award 2019.
Less

Prof. Jingying Zhou
Research Assistant Professor
School of Biomedical Sciences
The Chinese University of Hong Kong
Biographical Sketch
Biographical Sketch
Prof. Jingying ZHOU obtained her Ph.D. degree in Microbiology from the AIDS Institute, Department of Microbiology, The University of Hong Kong (HKU) in 2013 and received the Awards for Outstanding Research Postgraduate Student (HKU). She continued her research as a postdoctoral fellow and then promoted as in a Research Assistant Professor in 2018 at School of Biomedical Sciences. Prof. Zhou has published in international journals including Cancer research, Gut, Journal of Clinical Investigation, Nature Communications, Science Translational Medicine and served as co-inventor of two U.S. patents on DNA vaccines. Prof. Zhou’s research interests are mainly focused on cancer immunology and immunotherapy.
Less
Other CUHK Teachers

Dr. Daniel Lee
Associate Vice-President
(Innovation and Enterprise)
The Chinese University of Hong Kong
Former Head of Biomedical Technology Cluster
Hong Kong Science and Technology Parks Corporation
Biographical Sketch
Biographical Sketch
Dr. Daniel Lee is appointed as Associate Vice-President (Innovation and Enterprise) of The Chinese University of Hong Kong from 2019. Dr. Lee is a clinical biochemist and has been an Advisory Committee Member of the School of Pharmacy, and Scientific Advisor of the Clinical Trial Centre, Prince of Wales Hospital, for CUHK. He obtained his BSc and PhD in Pathology from The University of Hong Kong. After teaching at The University of Hong Kong for seven years, he joined the pharmaceutical industry and served for over 25 years in pharma companies including Johnson and Johnson USA, Biogen USA, GlaxoSmithKline (GSK) China and served as General Manager and Head of site for Roche Pharma R&D China from 2010. He has broad experience of both small molecule and biologic drug discovery and development, including companion diagnostics, covering therapeutic areas from central nervous system, infectious diseases, diabetes complications to oncology and inflammation. He joined the Hong Kong Science and Technology Parks Corporation as Head of the Biomedical Technology Cluster from 2013 to 2018 and contributed significantly to the biotech industry and translational medicine development in Hong Kong. He has published over 50 peer-reviewed papers, actively engaged in teaching and journal manuscript reviews, and co-invented 14 published patents. He has served as CEO and board director of biotech companies, consultant for several institutes and companies, Adjunct Professor of the Division of Life Science, The Hong Kong University of Science and Technology and Honorary Professor of the Faculty of Medicine, The University of Hong Kong.
Less

Prof. Wayne Lee
Assistant Professor
Department of Orthopaedics and Traumatology
The Chinese University of Hong Kong
Biographical Sketch
Biographical Sketch
Prof. Lee obtained his PhD in Pharmacology from The Chinese University of Hong Kong in 2009, then had postdoctoral training in the Department of Orthopaedics and Traumatology of CUHK. He has published over 75 publications in peer-reviewed international journals (Scopus H-index 27), and serves as reviewers of a number of international journals. His research can be divided into two major areas of orthopaedic with important clinical relevance. The first line focuses on the development of cell therapy to treat osteoarthritis and other musculoskeletal problems. His team also explore novel treatment strategies such as MSC secretome, miRNA and biomaterials with cell tracking and controlled release properties. The second line is on the biological roles of osteocyte in adolescent idiopathic scoliosis and bone aging. His team has developed advanced research platforms for the study of osteocyte, and recently established a composite model composed of circulating microRNA and bone turnover marker to predict curve progression.
Less

Prof. Chi Kong Li
Professor
Department of Paediatrics
The Chinese University of Hong Kong
Biographical Sketch
Biographical Sketch
Prof. Chi Kong Li graduated from the University of Hong Kong in 1981. He completed general paediatric training at Hong Kong and then specialised in paediatric/ haematology/ oncology/ BMT, with further training at John Radcliff Hospital, Oxford, and Great Ormond Street Hospital, London in 1986, and Fred Hutchison Cancer Research Centre, Seattle in 1992. Prof. Li joined Prince of Wales Hospital (PWH) in 1989 as Senior Medical Officer and became consultant in 1993, and is now a professor. Currently he is also honorary professor in 3 medical universities in China. His main interest is in childhood leukaemia, haematopoietic stem cell transplantation and thalassaemia. He is the study chair for acute lymphoblastic leukaemia trials in Hong Kong and also local coordinators for several international multicenter studies. He introduced the new treatment methods for Hong Kong children including double unit umbilical cord blood transplantation, Metaiodobenzylguanidine (MIBG) treatment for neuroblastoma. Prof. Li also actively involves in the development of paediatric oncology in mainland China. He is vice chairman of China Children Cancer Group, and vice-chairman of steering committee for two consecutive multi-center leukaemia studies in China. Currently he is the President of Asia Continent of International Society of Pediatric Oncology. In recent years he also actively involved in the development of children’s palliative care in Hong Kong and mainland China. He has published over 300 peer-reviewed papers, written chapters in 4 books and editor of one book. He also serves as editors in medical journals including Pediatric Blood Cancer, Chinese Journal of Pediatrics, China Journal of Pediatric Hematology & Oncology.
Less
Experts from Various Fields
Drug Office, Department of Health, The Government of HKSAR

Mr. Raccoon Chung
Senior Pharmacist
Drug Office
Department of Health
The Government of HKSAR
Biographical Sketch
Biographical Sketch
Mr. Raccoon Chung has been working in the Drug Office of the Department of Health for more than 10 years. He obtained his Bachelor degree of Pharmacy from the Chinese University of Hong Kong, and his Master degree in Clinical Pharmacy and in Regenerative Medicine from the University of Sunderland and the University of Edinburgh respectively. He also obtained his Bachelor and Master degrees of Laws from the University of London. Mr Chung has a working experience in the Pharmacovigilance Unit and Manufacturers Regulatory Unit of the Drug Office before and he was a qualified GMP inspector. In recent years, Mr Chung engaged in preparing guidance on the proposed regulatory framework of Advanced Therapy Products in Hong Kong. Mr Chung is now working in the Adverse Event Following Immunization Team of the Drug Office.
Less

Ms. Kim Ho
Pharmacist (Manufacturers Regulatory)
Drug Office
Department of Health
The Government of HKSAR
Biographical Sketch
Biographical Sketch
Ms Kim Ho is a registered pharmacist and is currently a GMP inspector of Manufacturers Regulatory Unit at Drug Office, Department of Health. Her main duties include conducting GMP inspections on pharmaceutical manufacturers, making assessment on application for change of particulars from manufacturer licensees and evaluating new application of license for manufacture of pharmaceutical products. She is specialized in the inspection of sterile pharmaceutical and advanced therapy products manufacturing. She had also participated in preparing the guidance for industry published by Drug Office on the interpretation of the Pharmaceutical Inspection Co-operation Scheme (PIC/S) Guide to Good Manufacturing Practice (GMP) for Medicinal Products.
Less
Other Experts

Ms. Gigi Cham
Quality and Regulatory Affair Director
Powder Pharmaceuticals, Inc.
Biographical Sketch
Biographical Sketch
Ms. Gigi Cham obtained her Bachelor degree in Food Science from the University of California, Davis and her Master degree in Pharmaceutical Manufacturing and Quality (PMQ) from the Chinese University of Hong Kong. She has been certified by the American Society for Quality as the Quality Auditor and Pharmaceutical GMP Professional.
Prior returning to Hong Kong, Ms. Cham worked in several US pharmaceutical companies such as Johnson & Johnson to support the analysis and production for drug products and medical devices that have obtained the approval of various global regulatory agencies. She has acquired various experience in several drug delivery technologies, including transdermal, implantable, intravenous, and electro-transport.
Ms. Cham is currently the Quality and Regulatory Affairs Director of a local manufacturer, leading the quality and regulatory functions and serving as management representative. Her role focuses on optimizing quality, ensuring regulatory compliance, and reducing risk through implementation of company’s policy and standard operating procedures. Furthermore, she is responsible for new product registration in the global market such as US, China, Singapore, United Arab Emirates (UAE), European Union (EU), Australia, etc., to support product expansion.
Less

Dr. Nelson Chan
Associate Consultant
Department of Pathology
Hong Kong Children’s Hospital
Biographical Sketch
Biographical Sketch
Dr. Nelson Chan obtained his Bachelor of Medicine and Bachelor of Surgery from the Chinese University of Hong Kong in 2008. He has multiple professional affiliations, including the Royal College of Physicians, the Royal College of Pathologists, the Hong Kong College of Physicians, the Hong Kong College of Pathologists and the Hong Kong Academy of Medicine. He is also a member of the International Society of Cellular and Gene Therapy. Dr. Chan has published in multiple peer-reviewed haematology journals, including Blood, the British Journal of Haematology and the American Journal of Haematology. In 2019, he was awarded the Cooperate Scholarship of the Hospital Authority to undergo a 6 months training in GMP manufacture of advanced therapeutic medicinal products (ATMP) in the John Goldman Center of Cellular Therapy, Imperial College London. He also attended the CAR-T preceptorship of the King’s College London. His research interest include inherited red cell disorders, stem cell transplantation and advanced therapeutic medicinal products.
Less

Dr. Paul Cheng
Medical Oncologist
Co-Founder & Executive Director of
Bio-Cancer Treatment International Ltd.
Biographical Sketch
Biographical Sketch
Dr. Paul Cheng who is a practicing medical oncologist and haematologist, founded BCT (Bio-Cancer Treatment Ltd). Dr. Cheng has been an Adjunct Associate Professor of Applied Biology and Chemical Technology of the Hong Kong Polytechnic University and was on the faculty of medical school of University of Hong Kong and the medical school of Chinese University of Hong Kong. He has extensive experience in research as well as clinical trials and is an author of over 30 publications in medical journals as well as a chapter of a textbook on oncology. His current research interests are arginine restriction in cancer therapy and cycle signaling control in cancer. Dr. Cheng graduated with MBChB in 1979 from the University of Wales College of Medicine where he also obtained his haematology training and his MD. Dr. Cheng obtained his medical oncology training at Memorial Sloan-Kettering Cancer Center in the U.S.
Less

Dr. Abby Yuanwei Gao
Director of Investment Research
DEFTA Limited
Biographical Sketch
Biographical Sketch
Dr. Abby Yuanwei Gao is the Director of Investment Research at DEFTA Limited. Before she joined DEFTA, she was the former manager of BioMedical Technology Cluster (Therapeutics) in Hong Kong Science and Technology Parks Corporation promoting development and commercialization of innovative technology in therapeutic area and facilitating the business development of biotech companies. She received her bachelor’s degree from Tsinghua University (Beijing, China) and her Ph.D. in Barnett Institute from Northeastern University (Boston, MA, U.S.A.). Prior to join HKSTP, she worked in Merck Sharp & Dohme as a senior scientist focusing on pharmacokinetics, pharmacodynamics and drug metabolism for drug discovery in the U.S.. During her study and working time in pharmaceutical industry, she has accumulated lots of experiences about drug discovery and development, biologics CMC and regulatory development. In the collaboration with Bristol-Myers Squibb, she optimized the upstream bioprocess through the study of proteome of production CHO cell lines used in the large-scale production and developed the mass-spectrometry based host cell protein detection for downstream purification of therapeutic protein products, improving the industrial biopharmaceutical production.
Less

Dr. Hovhannes Gukasyan
Associate Professor of Pharmacology and
Pharmaceutical Sciences
School of Pharmacy
University of Southern California (USC)
Biographical Sketch
Biographical Sketch
Dr. Hovhannes J. Gukasyan (Hovik) obtained his PhD from University of Southern California (USC), School of Pharmacy, Department of Pharmaceutical Sciences in 2004. 18 years in pharma industry, he is currently the technology and CMC lead consultant at 6 different small- to medium-sized biotech firms. He is an expert in pharmaceutical R&D contributions in development, characterization, and application of drug delivery systems/technologies in novel/early clinical studies. He supported >80 ‘Pipeline’ programs, at Pfizer Inc and Allergan plc, with successful examples marketed as Diquas®, Vyzulta®, Lorbrena®. His contributions included enabling development of new chemical entities (NCE), new biological entities (NBE), nonbiological complex drugs (NBCD) using molecular biopharmaceutics and digital modeling & simulation sciences. He recently joined the University of Southern California (USC) School of Pharmacy as Associate Professor of Pharmacology and Pharmaceutical Sciences.
Less

Ms. Lorita Ho
Assistant Director – Corporate Quality
New Beta Innovation Ltd.
Biographical Sketch
Biographical Sketch
Ms. Lorita Ho is currently Assistant Director – Corporate Quality at New Beta Innovation Ltd. with oversight of the Quality Units (Quality Assurance (QA), Quality Control (QC) and Compliance) at the company’s biopharmaceutical GMP manufacturing sites in Hong Kong and Canada. These units are important in ensuring the sterile biologics manufactured meet all relevant international quality standards and regulatory requirements. Educated as a pharmacist, Ms. Ho always puts patients’ safety and well-being as her highest priority. That motivated Ms. Ho to join the pharmaceutical manufacturing sector in 2006, focusing on the quality and regulatory aspects of medicine. In the present training program, Ms. Ho aims to share her experience in adopting evolving quality guidelines and regulatory requirements such as those in Pharmaceutical Inspection Co-operation Scheme (PIC/S) and International Council on Harmonisation (ICH), with an emphasis on the applications of Quality Risk Management and its integration into Pharmaceutical Quality System in advanced therapy products.
Less

Ms. Gloria Hung
Head of North Asia Cluster
Head of Regulatory Affairs Hong Kong
Global Regulatory Affairs – International
Pfizer Corporation Hong Kong Ltd.
Biographical Sketch
Biographical Sketch
Ms. Gloria Hung has contributed to local, regional and global regulatory strategy and operations at Pfizer since 2006. She currently oversees Regulatory Affairs of North Asia Cluster. She manages the country teams to attain portfolio expansion and life cycle management. Her previous role was the Head of the Asia Regional Regulatory Strategist Hub where she set up the first virtual Asia regional team liaising strategy between local and global stakeholders. Prior to joining Pfizer, She was the Authorized Person of Jean-Marie Pharmacal Company, Hong Kong.
Ms. Hung is a pharmacist by training and obtained her Bachelor of Pharmacy from the University of Nottingham, England. She then practised in Ipswich Hospital (NHS), England before studying prostaglandin EP3 receptor pharmacology for her MPhil at The Chinese University of Hong Kong. She has also obtained Postgraduate Diploma of Public Health from the University of Hong Kong.
Less

Ms. Cindy Ko
Quality Assurance Manager
Authorized Person
Fortune Pharmacal Co., Ltd.
Biographical Sketch
Biographical Sketch
Ms. Cindy Ko is currently the Quality Assurance (QA) Manager and the Authorized Person in Fortune Pharmacal Co., Ltd. Ms. Ko is a registered pharmacist who obtained her Bachelor Degree in Pharmacy from the Chinese University of Hong Kong. She also obtained a Master Degree in Clinical Pharmacy from the University of Sunderland and a Master Degree in Pharmaceutical Manufacturing & Quality. Ms. Ko has joined the pharmaceutical manufacturing industry since 2010 and she focused on the areas of quality assurance and regulatory inspection. Being the QA Manager, Ms Ko ensures the establishment and implementation of Quality Management System effectively. Besides, as the Authorized Person of the company, Ms. Ko is responsible to certify that a product has been manufactured in accordance to the registered particulars and with Good Manufacturing Practice (GMP), and she plays an important role to lead different departments to undergo GMP inspection. With the experience in pharmaceutical manufacturing in Hong Kong, Ms. Ko will share her experience in implementation of the Quality Management System (QMS) according to Pharmaceutical Inspection Co-operation Scheme (PIC/S) and Hong Kong regulatory requirement.
Less

Dr. Jim Lu
Co-founder and Chief Scientific Officer (CSO),
TriArm Therapeutic Co.
Biographical Sketch
Biographical Sketch
Dr. Lu is the co-founder and CSO of TriArm Therapeutic Co., a company specializing in developing innovative cellular immunotherapies for unmet medical needs. Prior to joining TriArm, he worked at US FDA as an expert reviewer at OTAT, CBER, FDA. He was a recognized expert in cell and gene therapy field and coauthored several FDA Guidance for Industries. Dr Lu served on the scientific committee of Oncology Center of Excellence, FDA and was the recipient of the CBER award for Excellence in Regulatory Science Research in 2015.
Prior to joining the FDA, Dr Lu worked in the biotechnology industry as a senior scientist, manager and consultant in the development of viral vaccines and of viral vectors for cancer therapy.
Less

Dr. Cindy Ru
Managing Director
CRC Oncology, San Diego, U.S.A.
Biographical Sketch
Biographical Sketch
Dr. Qinhua Cindy Ru, Ph.D., is currently the Managing Director of CRC Oncology, San Diego U.S.A. Before that, she was the Chief Scientific Officer and Executive Vice President at CARsgen Therapeutics, Ltd in 2017. Dr. Ru served as Chief Scientific Officer and Assistant to the Chairman & General Manager of Clinical Center at Sihuan Pharmaceutical Holdings Group Ltd. since 2016 until March 4, 2017. Dr. Ru joined Sihuan Pharmaceutical Holdings in 2016. Before joining Sihuan, Dr. Ru holds near 20 years of indepth international working experience in Oncology preclinical research, clinical research and development, global regulatory registration, global product launch and asset life cycle management. From 2003 to 2006, Dr. Ru was a Principal Scientist working at Windber Research Institute, a breast cancer clinical research institution cofounded by Walter Reed Army Medical Center (Washington DC) and Windber Medical Center (Windber PA). Dr. Ru was a leading sub-investigator in the Clinical Breast Care Project (CBCP), a breast cancer vaccination development program sponsored by the US Department of Defense. From 2006 to 2007, she served as a clinical research scientist in the Oncology Clinical Department at Merck & Co., Inc. (Upper Gwynedd PA). From 2007 to 2013, Dr. Ru served as the Global Clinical Trial Head in Novartis Oncology Clinical Department (Florham Park NJ) and efficiently led a global cross-functional team completed multiple clinical trials of a drug candidate and successfully brought the drug from early phase to late phase development. From 2013 to 2014, She was the Senior Director at Exelixis (South San Francisco CA) and clinical science lead of METEOR, a randomized pivotal Phase III trial of cabozantinib versus everolimus in patients with advanced renal cell. Between 2014 and 2016, Dr. Ru served as the Senior Director at Pfizer Oncology Late Phase Clinical Development (La Jolla CA) and was the Hematology Asset Team Lead and “CEO” of inotuzumab ozogamicin Global Asset Team, oversaw the late phase clinical development, global regulatory submission, global product launch and global asset life cycle management strategy of antibodydrug-conjugates (ADCs). She completed the first-in-man trial significantly ahead of timeline and was the winner of Novartis President Award, managing multiple global phase II PoC trials and Phase III pivotal trial of burparlisib (BKM120) in Breast Cancer, Glioblastoma and NSCLC for consecutive 6 years. Dr. Ru acquired her Ph.D. degree in Chemistry from Tsinghua University (Beijing, China) in January 2001. She further completed her Post-Doctoral training in the US Department of Defense sponsored Los Alamos National Laboratory Bioscience Division (Los Alamos, NM) in 2003.
Less
(In alphabetical order.)

Contact |
|
| Please feel free to contact us for further enquiry about the course. | |
| Address: | School of Pharmacy 8th Floor, Lo Kwee-Seong Integrated Biomedical Sciences Building, Area 39, The Chinese University of Hong Kong, Shatin, N.T., Hong Kong |
| Tel: | 852-39436862 |
| Fax: | 852-26035295 |
| Email: | atp@pharmacy.cuhk.edu.hk |
| Course Leaflet: | Link |
Remark: School of Pharmacy reserves the right on the cancellation of courses, amendment of program structure and change of teaching faculty.
PHAR5000 Regulation
Regulation of Advanced Therapy Products 先進療法產品規管
Objectives
This course is designed to provide an overview of the regulatory requirements of the Pharmacy and Poisons Board of Hong Kong (PPB) and other jurisdictions. The paramount importance of ethics and intellectual property in ensuring drug product quality will be emphasized. Equally important is the role, training, and responsibilities of the qualified person. The central importance of risk-based regulatory decision making, and International Council on Harmonization (ICH)/Pharmaceutical Inspection Co-operation Scheme (PIC/s) quality guidelines in the quality assurance of advanced therapy products will be emphasized.
Expected Learning Outcome
Upon completion of this course, students will have acquired the ability to:
| 1. | Understand the central importance of data integrity and intellectual property in commercial drug development |
| 2. | Know the scope of advanced therapy products and the key steps in their manufacture and registration |
| 3. |
Understand how Hong Kong’s regulatory framework is similar to, and different from, those of European Medicines Agency (EMA) and U.S. Food and Drug Administration (FDA)
|
| 4. | Understand the justifications for innovative clinical trial design |
Course Structure
| Proposed Date of Commencement: | July 2021 |
| Duration of the Course: | 3 months (40 hours) |
| Language: | English |
Application
| Target Participants: | Graduates or postgraduates in pharmacy, pharmaceutical science, engineering, law, nursing, medicine, Chinese medicine, life science, biomedical science, chemistry, and with course director’s consent, fine arts, journalism, and mathematics |
| Course Fee: |
|
| Application Deadline: | 20 May 2021 |
| Application Link: | Closed |
PHAR5010 Scientific Rationale
Scientific Rationale of Advanced Therapy Products 先進療法產品的科學原理
Objectives
This course provides an overview on the scientific rationale that underlies the clinical applications of advanced therapy products. Focuses will be on the applications of immunotherapy and stem cell transplant in treatment of cancer, two areas of mature development. The patient populations likely to benefit from personalized medicine and in turn companion diagnostics.
Expected Learning Outcome
Upon completion of this course, students will have acquired the ability to:
| 1. | Understand the cell biology and genomic rationale of ATP |
| 2. | Understand the parallel application of ATP in personalized medicine and development of orphan drugs |
| 3. | Understand the design, development, efficacy and safety evaluation of CAR-T cells in approved and expanding indications |
| 4. | Understand the design, development, efficacy and safety evaluation of bone marrow stem cell transplants in cancer treatment |
Course Structure
| Proposed Date of Commencement: | October 2021 |
| Duration of the Course: | 3 months (60 hours/ 40 hours)
Students can choose either option (A) or (B):
*Laboratory section of the course is a pre-requisite of “PHAR5032 Hands-on” course. Students who plan to take PHAR5032 should choose option (A). |
| Weekly Schedule: |
|
| Teaching Mode: | Face-to-face lectures & lab sections at CUHK Campus |
| Language: | English |
| Assessment Methods: |
|
| Graduation Requirement: | Students must fulfill all of the following criteria to be granted a certificate of completion issued by the School of Pharmacy:
For students who achieve 75% attendance, but do not pass the assessment, a certificate of attendance will be issued instead. |
Application
| Target Participants: | Graduates and postgraduates in pharmacy, pharmaceutical science, life science, biomedical science, clinical science, engineering, and chemistry who are contemplating a career in the biopharmaceutical industry, and who are already engaged in the manufacture and production of ATP but who wish to have a refresher in the basic science and rationale underpinning the manufacture of quality ATP. |
| Entry Requirement: | Applicants should fulfill the following requirements,
|
| Proposed Intake: | Up to 30 students (Limited quota for option (A), first-come-first-served) |
| Course Fee: |
|
| Enrolment: | Applicants should complete an online application form and upload copies of supporting documents on the online registration system. Applicant should also pay both the application fee of HK$300 and tuition fee of HK$45,000/ HK$30,000. Applications will be processed only upon receipt of the completed application form, copies of supporting documents, application fee and tuition fee, by the application deadline.
We will confirm applicants about enrolment status by email no later than two weeks prior to course commencement. For applicants who are not admitted to the course, tuition fee will be refunded accordingly, while the application fee of HK$300 is non-refundable. Note to non-local applicants:
|
| Application Deadline: | 13 September 2021 |
| Application Link: | Closed |
PHAR5020 Manufacture
Manufacture of Advanced Therapy Products 先進療法產品製造
Objectives
This course focuses on important aspects of Good Manufacturing Practice (GMP) from International Conference on Harmonization (ICH) and Pharmaceutical Inspection Co-operation Scheme (PIC/s) perspectives. These aspects include process validation, analytical method validation, instrument validation, supply chain planning, documentation, regulatory dossiers, out of specifications (OOSs), and corrective action preventive action (CAPA), and post approval changes. Aspects related to microbial contamination, clean room design, facility requirements, and product stability will also be discussed. Aspects unique to the manufacture of products in gene therapy, cell therapy and regenerative medicine will be elaborated upon.
Expected Learning Outcome
Upon completion of this course, students will have acquired the ability to:
| 1. | Apply concepts of GMP integral to process validation, analytical method validation, instrument validation, supply chain planning, documentation, regulatory dossiers, OOSs, CAPA, and post approval changes to the manufacture of ATP. |
| 2. | Understand aspects related to microbial contamination, cleanroom design, and facilities design for ATP manufacture |
| 3. | Understand aspects unique to the manufacture of products in gene therapy, cell therapy, and regenerative medicine |
Course Structure
| Proposed Date of Commencement: | January 2022 |
| Duration of the Course: | 3 months (40 hours) |
| Weekly Schedule: |
|
| Teaching Mode: |
Face-to-face lectures at CUHK Campus (some lectures may be arranged online via ZOOM subject to the availability of the overseas guest speakers) |
| Language: | English |
| Assessment Methods: |
|
| Graduation Requirement: |
Students must fulfill all of the following criteria to be granted a certificate of completion issued by the School of Pharmacy:
For students who achieve 75% attendance, but do not pass the assessment, a certificate of attendance will be issued instead. |
Application
| Target Participants: |
Graduates and postgraduates in pharmaceutical science, life science, biomedical science, clinical science, engineering, and chemistry who are contemplating or already engaged in the manufacture and production of ATPs. |
| Entry Requirement: |
Applicants should fulfill the following requirements,
|
| Proposed Intake: | Up to 30 students |
| Course Fee: |
|
| Enrolment: |
Applicants should complete an online application form and upload copies of supporting documents on the online registration system. Applicant should also pay both the application fee of HK$300 and tuition fee of HK$30,000. Applications will be processed only upon receipt of the completed application form, copies of supporting documents, application fee and tuition fee, by the application deadline. We will confirm applicants about enrolment status by email no later than two weeks prior to course commencement. For applicants who are not admitted to the course, tuition fee will be refunded accordingly, while the application fee of HK$300 is non-refundable. Note to non-local applicants:
|
| Application Deadline: | 17 December 2021 |
| Application Link: | Closed |
PHAR5030 Quality Control
Quality Control of Advanced Therapy Products 先進療法產品的質量控制
Objectives
This course focuses on important aspects of Good Manufacturing Practice (GMP) from International Conference on Harmonization (ICH) and Pharmaceutical Inspection Co-operation Scheme (PIC/s) perspectives. These aspects include process validation, analytical method validation, instrument validation, supply chain planning, documentation, regulatory dossiers, out of specifications (OOSs), and corrective action preventive action (CAPA), and post approval changes. Aspects related to microbial contamination, clean room design, facility requirements, and product stability will also be discussed. Aspects unique to the manufacture of products in gene therapy, cell therapy and regenerative medicine will be elaborated upon.
Testing the finished product repeatedly to identify defective cohorts for rejection is costly. This provides the impetus for an alternative scientifically rigorous approach whereby quality is designed into the product. This so-called quality by design approach (QbD) results from integrating the principles that underpin International Conference on Harmonization (ICH) Q8, Q9, and Q10. Assays that will confirm the physicochemical integrity of the product and its ultimate biological activity, as defined by ICH Q14, play an important role in the success of QbD. Aspects of infrastructure and involvement of corporate leadership in creating and sustaining a quality culture will be emphasized.
Expected Learning Outcome
Upon completion of this course, students will have acquired the ability to:
| 1. | Understand the role difference between quality assurance and quality control |
| 2. | Apply Quality by Design (QbD) to build quality into the product |
| 3. | Understand the Pharmaceutical Inspection Co-operation Scheme (PIC/s) requirements in quality control |
| 4. | Understand the active role of ICH requirements in modernizing quality control |
Course Structure
| Proposed Date of Commencement: | April 2022 |
| Duration of the Course: | 3 months (40 hours) |
| Weekly Schedule: |
|
| Teaching Mode: | Lectures are tentatively arranged via online ZOOM * (subject to University’s latest announcement)
*Format of the final examination has yet to be confirmed (Face-to-face on campus or online) |
| Language: | English |
| Assessment Methods: |
|
| Graduation Requirement: | Students must fulfill all of the following criteria to be granted a certificate of completion issued by the School of Pharmacy:
For students who achieve 75% attendance, but do not pass the assessment, a certificate of attendance will be issued instead. |
Application
| Target Participants: | Graduates and postgraduates in pharmaceutical science, life science, biomedical science, clinical science, engineering, and chemistry who are contemplating or already engaged in the manufacture and production of ATPs. |
| Entry Requirement: | Applicants should fulfill the following requirements,
1. Graduated from a recognized university/tertiary educational institution with a bachelor’s degree or above in pharmacy, pharmaceutical science, life science, biomedical science, clinical science, engineering, chemistry, or related field. 2. Preferably having 2 or more years of experience in the pharmaceutical industry or life science/chemistry laboratory |
| Proposed Intake: | Up to 30 students |
| Course Fee: |
|
| Enrolment: | Applicants should complete an online application form and upload copies of supporting documents on the online registration system. Applicant should also pay both the application fee of HK$300 and tuition fee of HK$30,000. Applications will be processed only upon receipt of the completed application form, copies of supporting documents, application fee and tuition fee, by the application deadline.
We will confirm applicants about enrolment status by email no later than two weeks prior to course commencement. For applicants who are not admitted to the course, tuition fee will be refunded accordingly, while the application fee of HK$300 is non-refundable. Note to non-local applicants:
|
| Application Deadline: | 18 March 2022 |
| Application Link: | Closed |
PHAR5032 Hands-on
Hands-on Good Manufacturing Practice Training 良好生產規範的實踐培訓
Objectives
This course is designed to provide in-depth hands-on training of key Current Good Manufacturing Practice (cGMP) concepts that are the foundation of procedure, process, environment, and personnel training in the assurance of producing pharmaceutical products of consistent quality.
Expected Learning Outcome
Upon completion of this course, students will have acquired the ability to:
| 1. | Apply the fundamentals of cGMP to manufacture ATP of consistent quality |
| 2. | Understand the importance of technical writing comprising standard operating procedures and assignment of responsibility for departmental procedures |
| 3. | Apply cGMP procedures integral to Pharmaceutical Inspection Co-operation Scheme (PIC/s) compliance and pharmaceutical quality control, including root cause investigations for Corrective and Preventive Action (CAPA) and packaging of pharmaceutical products |
Course Structure
| Proposed Date of Commencement: | Coming soon |
| Duration of the Course: | 2 months (20 hours) |
| Language: | English |
Application
| Target Participants: | Graduates or postgraduates in pharmacy, pharmaceutical science, life science, biomedical science, and engineering who are committed to a career in pharmaceutical manufacturing, quality assurance, or quality control. |
| Course Fee: |
|
| Application Link: | Coming Soon |


