Dear readers, With the launch of e-newsletter CUHK in Focus, CUHKUPDates has retired and this site will no longer be updated. To stay abreast of the University’s latest news, please go to https://focus.cuhk.edu.hk. Thank you.
A Leg-up to Stroke Survivors
Vincent Cheung unveils muscle synergies that make us move

A human movement such as running requires an incredibly complex network of neurons to fire in coordination. Our brain must translate the intention to move into suitable commands to neurons in the motor system that in turn signal how the hundreds of muscles in the body should move.
The sheer output of information would pose a serious challenge to, say, the computational power of our devices. But the body needs to do it on the spot.
Prof. Vincent Cheung brings a mathematician’s precision and a penchant for hard data to his analysis of neuroscience. His work helps understand these human muscle synergies—the neural networks that simultaneously activate a set of muscles to enable movement—in a way that ultimately may enable, say, stroke victims to learn to walk again.
The work on muscle synergies may also help a wide spectrum of nervous-system disorders that involve muscular atrophy or the degeneration of motor neurons. His collaborators are also working to combat cerebral palsy, Parkinson’s disease and ‘Lou Gehrig’s disease’, or amyotrophic lateral sclerosis, the inspiration behind the ‘ice-bucket challenge’.
‘You can engineer how a patient can generate movement by changing their muscle synergies,’ Professor Cheung says. ‘In a stroke survivor, a lot of the motor impairment comes from their abnormal muscle synergies.’
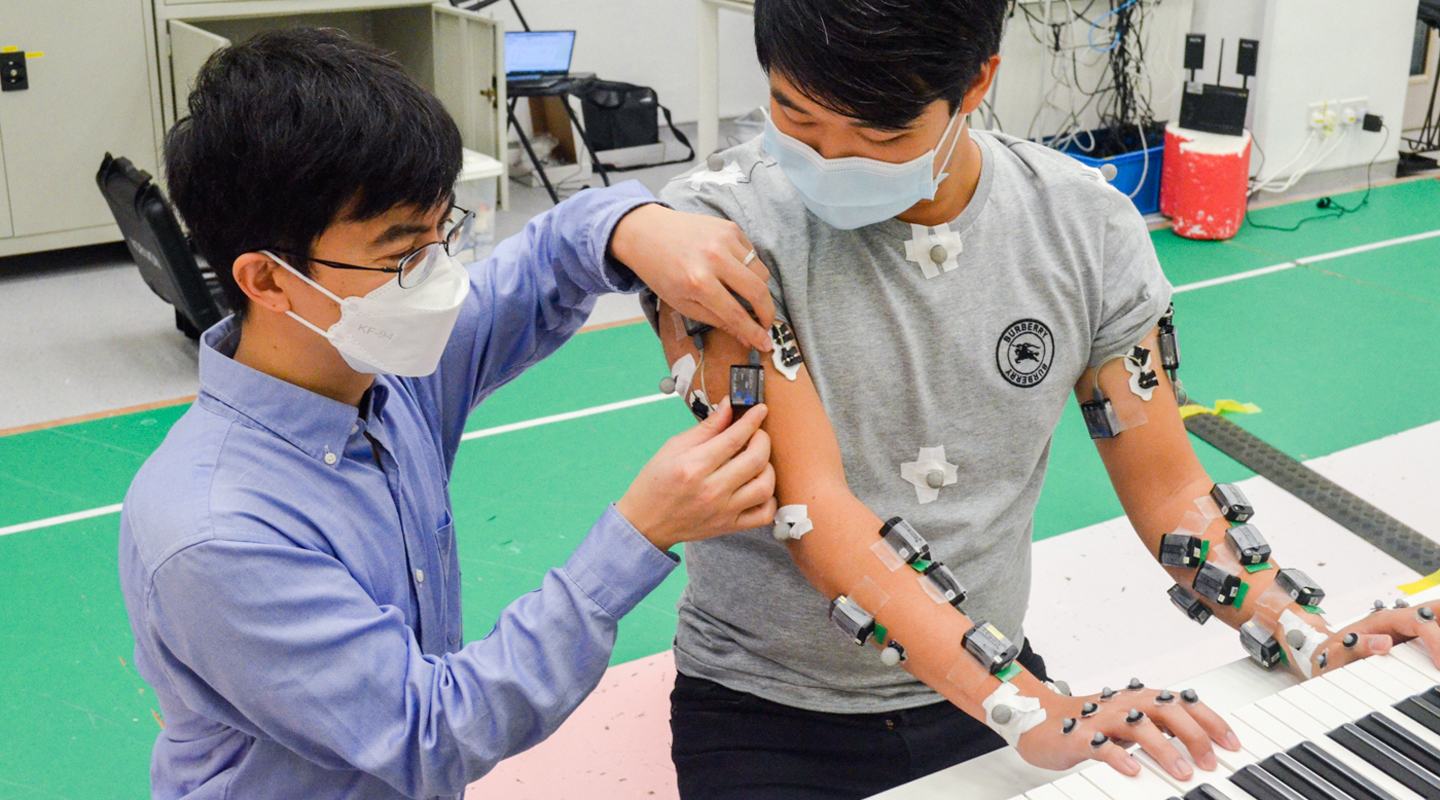
Professor Cheung hopes to usher in a whole new world of ‘precision rehab’ that would teach stroke victims how to maximize their movement once again. He wants to bring stroke patients into his lab, where they would walk on a treadmill while wearing a safety harness and a device that would deliver small electric stimuli to muscles in the stroke-affected leg. This noninvasive process, known as functional electrical stimulation, should prompt the stroke survivor to re-learn the normal muscle synergies missing in their repertoire of movement.
‘We are explicitly guiding the nervous system to adopt a certain pattern of muscle activation, down to the level of muscles,’ Professor Cheung, an assistant professor in the School of Biomedical Sciences, says. ‘This stroke survivor may have muscle synergies A and B missing, and other stroke survivors may have C and D missing. It’s not only precise, it’s also personalized, depending on the goals and the impairment.’
In a recent paper in the journal Nature Communications, Professor Cheung and his team demonstrate that muscle synergies appear to undergo changes during child-to-adult development and during motor training. These changes are underpinned by two mechanisms: by splitting a pre-existing muscle synergy into multiple synergies with fewer muscles, a process known as fractionation, as well as by merging multiple synergies together into a unit with more muscles, a process called merging.
Over the years of our physical development and also as we train the body in various behaviours, our central nervous system gradually reorganizes our movement like an alphabet. This explains how we are able to add new physical skills in adulthood such as learning to swim, or to play a musical instrument.
For stroke victims, rehabilitation is currently most effective within six months of the stroke. After a year, many existing interventions provide only small benefit for a typical patient. If successful, the ‘precision rehab’ approach to target muscle synergies that are lacking or misfiring could be particularly beneficial for chronic stroke survivors who are no longer seeing benefits from traditional rehab.

The coronavirus has delayed the process of recruiting stroke patients to the study. For the time being, all non-vital research on human subjects has been put on pause. But Professor Cheung and his team have already collated baseline data from healthy individuals.
‘Hopefully next year we can have the first trial in a real stroke survivor,’ Professor Cheung says. ‘We are probably about half a year to 1 year behind.’
The combination of applied sciences with theoretical works appeals to Professor Cheung. He started his academic career studying pure mathematics and pharmacology before flirting with medical school and then going on to get a joint doctorate at MIT and Harvard Medical School in neuroscience and biomedical engineering.
‘Life is characterized by movement—we know we are alive because we move,’ Professor Cheung says. ‘From a more philosophical perspective, understanding how we move voluntarily is a key to understanding life in general.’
Yet medical science still knows little about the neural network, compared with other systems of the body. The science started with the physical dissection of cells and neurons to understand how they fire. That has given way to bigger questions surrounding highly complex neural circuits, a study that looks closer to computational sciences than biology.
He likens neuroscience now to the situation facing 19th century physics just before quantum physics and the theory of relativity. ‘We have not even scratched the surface. We might not even be asking the right questions,’ Professor Cheung says. ‘I think we are going to make a lot of breakthroughs.’

For instance, there are efforts under way to develop a ‘connectome’ of the nervous system of organisms, something akin to the wiring on a computer. But it is extremely challenging, several orders of magnitude more complex than sequencing the genome, for instance. There are an estimated 86 billion neurons in the brain alone, connected by an even greater number of synapses.
It is uncertain whether a connectome, which will be extremely challenging and costly to compile, would best be used. As an experiment, a team at Northwestern University sought to use the tools of neuroscience to understand the inner workings of a microprocessor, as if it were a brain. Although the physical network of a microprocessor is engineered, and therefore perfectly known, neuroscientists were unable to use their standard tools to create meaningful understanding of the microprocessor.
‘Neuroscience is in a transitional phase,’ Professor Cheung says. ‘We more or less know the limitations of existing technology, but there is in general a lack of ideas and theories about how to proceed. We are a little bit desperate waiting for new technologies, and we are awaiting a paradigm-setting idea.’
By Alex Frew McMillan
Photos by Eric Sin