CUHK
News Centre
CUHK Unlocks the Mystery of Small Heat Shock Protein Using Cryo-EM Technology Paves the Way for Plant Genetic Engineering
A research team from the School of Life Sciences at The Chinese University of Hong Kong (CUHK) has uncovered the anti-aggregation mechanism of small heat shock proteins (sHsps) and unveiled the structure of sHsps for the first time using the state-of-the-art single particle cryo-electron microscopy (cryo-EM) technology. The findings, recently published in the prestigious scientific journal Nature Communications, provide opportunities for potential enhancement of thermo-tolerance in crop plants and improvement in crop production.
Environmental stresses, such as drought, salinity and extreme temperatures, cause over 50% of worldwide yield loss of major crops every year. There is a broad scientific consensus that climate change and global warming will significantly impact future agricultural and food productivity. Therefore, a comprehensive understanding of environmental stresses tolerance mechanisms in plants would be of benefit and essential to genetic modification of crops with the aim of achieving sustainable agriculture and food supply.
Elevated temperature is considered as one of the major environmental stresses that affects the metabolism and many physiological processes of plants and thus has a devastating impact on plant growth and development. In a non-stressed environment, proteins fold into a functional shape and structure in order to function correctly and control dynamic processes in living cells. However, under conditions of stress, for instance, when temperature rises, proteins will tend to unfold and aggregate. Plants have evolved various defense mechanisms such as the heat shock response to cope with environmental stresses.
sHsps represent a class of highly conserved molecular chaperones, meaning they widely exist in plants and animals, and the genetic difference across species is not significant. A molecular chaperone is defined as protein that helps another protein to acquire its functional form. sHsps are known as “housekeeping” proteins to prevent aggregation and unfolding from happening under heat stress condition. In plants, genetically modified production of sHsps has been shown to confer enhanced thermotolerance.
Structural elucidation of Hsp21 and its complex with a natural substrate
To explore and open up the applicability of sHsps in plant biotechnology, Professor Wilson Chun Yu LAU, Assistant Professor, School of Life Sciences at CUHK, and his research team set out to investigate the molecular mechanism of a plant sHsp, Hsp21, using a structural biology approach. They chose to focus on the Hsp21, a crucial sHsp that protects all photosynthesizing plants from heat stress.
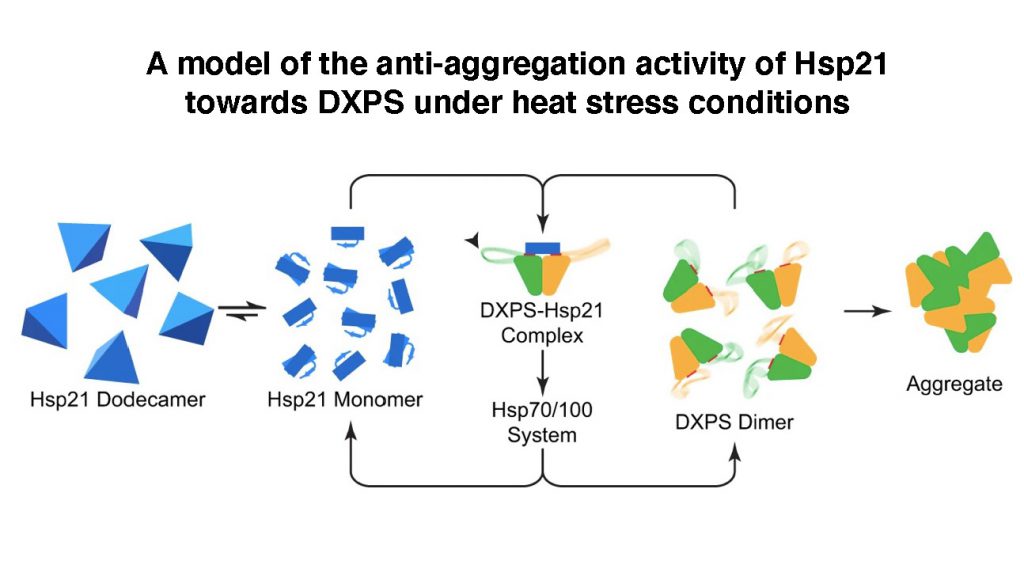
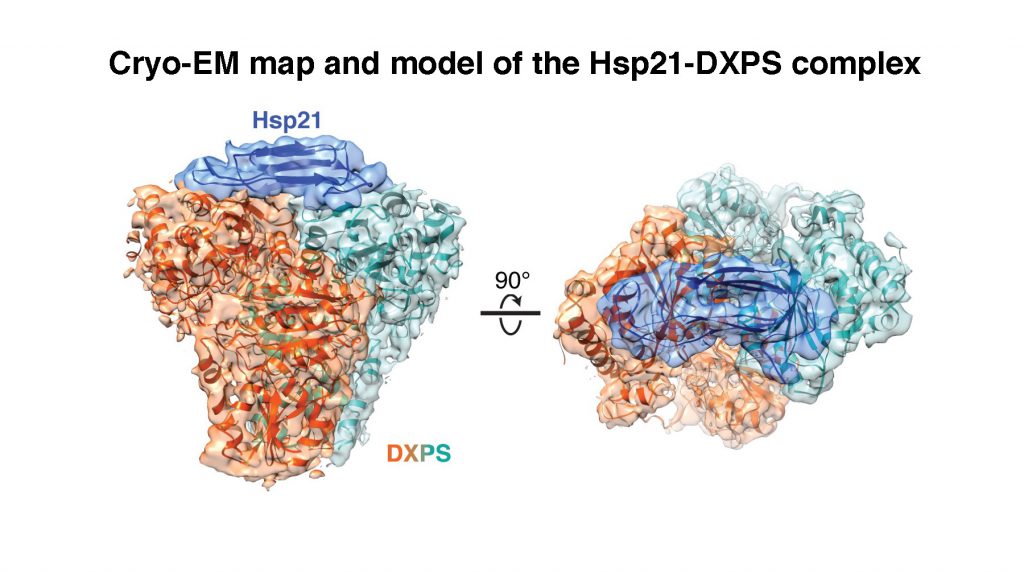
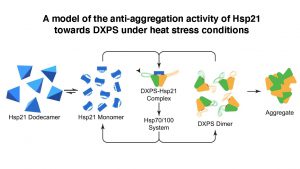
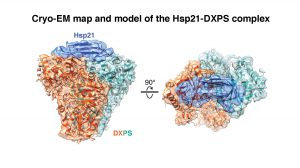
They first identified a substrate (a protein molecule upon which a chaperone acts on) of Hsp21, an enzyme called 1-deoxy-D-xylulose 5-phosphate synthase (DXPS), and then solved three-dimensional structures of Hsp21, DXPS and the Hsp21-DXPS complex, at unprecedented resolution, using cryo-EM of single particles combined with advanced computational image processing algorithms. Structural characterisation of sHsp-substrate complexes by the traditional X-ray crystallography method has proved notoriously difficult owing to the transient and heterogenous nature of their interactions.
Professor LAU said, “Through solving the Hsp21-DXPS structure, our work unravels an unanticipated mechanism of sHsps anti-aggregation activity that is likely applicable towards a wide range of substrates. The current work not only provides a structural framework for understanding the functional properties of Hsp21 and sHsps in general, but also could form a basis and provide reference for genetic engineering of heat-resistant food crops to fight global climate change.”
The study was carried out by Ms. Chuanyang YU, PhD student of Professor Wilson LAU, and Mr. Stephen King Pong LEUNG, and in collaboration with Professor Liwen JIANG from the School of Life Sciences at CUHK.
About Professor Wilson Chun Yu LAU
Professor Wilson Chun Yu LAU is a leading expert in cryo-EM and has made significant contributions to the studies of membrane proteins and macromolecular assemblies. Trained as a structural biologist, Professor LAU has gained invaluable, multidisciplinary research experience in internationally recognised laboratories during his graduate study at the University of Toronto, as well as postdoctoral research in Baylor College of Medicine, USA, and the University of Hong Kong. He was also awarded the prestigious AXA Research Fund Fellowship to support his later postdoctoral work. Professor LAU is the lead author in high profile publications in Nature and PNAS. His research is internationally recognised and has been recognised by F1000Prime.

(From left) Professor Wilson LAU, Research Assistant Mr. Stephen LEUNG, PhD student Ms. Chuanyang YU, in collaboration with Professor Liwen JIANG, have successfully uncovered the anti-aggregation mechanism of small heat shock proteins (sHsps) for the first time using the state-of-the-art single particle cryo-electron microscopy (cryo-EM) technology.