Home > Resources > Secondary School Corner > Resource Book for Sixth-Form Practical Chemistry (Photos - Experiment 11)

Resource Book for Sixth-Form Practical Chemistry
Experiment 11
Separation and Identification of the Major Components of Common Over-the-Counter Painkilling Drugs
 |
A quick-fit drying tube packed with anhydrous calcium chloride |
 |
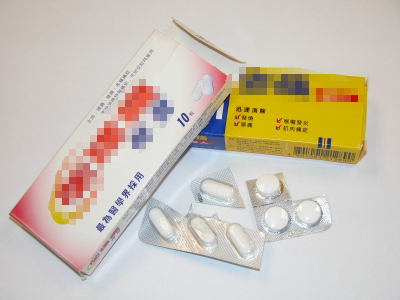
Painkilling tablets ground into fine powder |
 |
Separation of acetaminophen/binder from aspirin/caffeine: The tablet powder was suspended in dichloromethane and the suspension was warmed to about 30oC in a water-bath |
 |
|
 |
Separation of acetaminophen/binder from aspirin/caffeine: Filter the suspension Solid residue: acetaminophen and binder Filtrate: solution of aspirin and caffeine |
 |
The filtrate: The dichloromethane solution containing aspirin and caffeine |
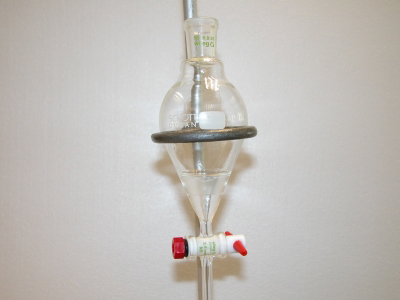 |
Separation of aspirin and caffeine by extraction: The separatory funnel Upper (aqueous) layer: aspirin dissolved in 3M NaOH solution Lower (organic) layer: caffeine dissolved in dichloromethane |
 |
|
 |
Organic layer: caffeine dissolved in dichloromethane Aqueous layer: aspirin dissolved in 3M NaOH solution |
 |
The aqueous solution containing aspirin was acidified with 3M HCl to about pH=2-3 |
 |
Aspirin was precipitated out from the acidified solution |
 |
Isolating the aspirin by suction filtration |
 |
The aspirin isolated from the painkilling tablet |
 |
Preparing standard sample solution for TLC analysis: Dissolving the standard samples in methanol |
 |
|
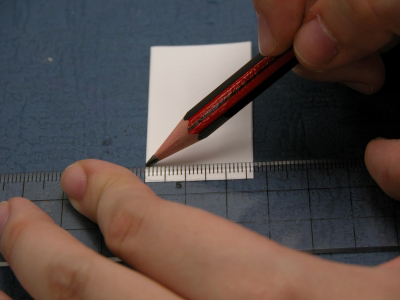 |
Marking the starting points on the TLC plate with a pencil |
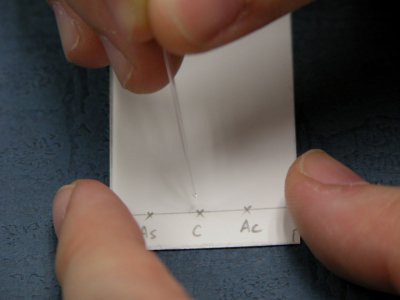 |
Spotting the sample solutions onto the TLC plate with a capillary tube |
 |
The TLC chamber and the eluting solvent for developing the TLC |
 |
Putting the TLC plate into the chamber carefully with a pair of forceps |
 |
Developing the TLC plate in the TLC chamber |
 |
|
 |
The iodine chamber for visualizing the colorless sample spots on the TLC plate |
 |
The spots of aspirin and acetaminophen display as yellow-brown spots on the TLC plate inside the iodine chamber left: aspirin middle: caffeine right: acetaminophen |
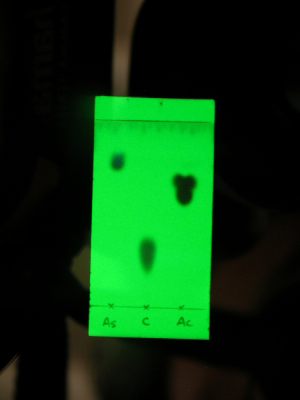 |
The sample spots visualized on the TLC plate under ultraviolet radiation left: aspirin middle: caffeine right: acetaminophen |
2004 Department of Chemistry, The Chinese University of Hong Kong


