News Centre
CUHK Uncovers Molecular Mechanism Regulating Blood-cerebrospinal Fluid Barrier Function in Developing Brain
New Direction for Treating Neurological Diseases
A research team from the School of Life Sciences at The Chinese University of Hong Kong (CUHK) has recently discovered that SOX9 protein is an essential regulatory factor of choroid plexus function that ensures the correct composition of cerebrospinal fluid (CSF). The finding, recently published in the prestigious scientific journal Proceedings of the National Academy of Sciences of the United States of America (PNAS), has provided the scientific community a novel understanding to the molecular regulatory mechanisms behind the function of the blood-CSF barrier and lays the groundwork for developing novel therapeutic strategies for preventing and treating neurodevelopmental disorders.
Dysfunction of blood-cerebrospinal fluid barrier is common in various neurological diseases
CSF is a clear, colourless body fluid that surrounds the brain and spinal cord, providing them a cushion against injuries. It also serves as a nutrient delivery and waste removal system for the brain. This major body fluid is produced and secreted by the regions of the choroid plexus. The choroid plexus consists of modified ependymal cells surrounding a core of capillaries and connective tissue. It filters the blood and restricts harmful molecules from entering into the central nervous system, thus forming a blood-CSF barrier that protects the nervous system and the brain.
Some studies have confirmed that multiple neurodevelopmental disorders such as autism and Alzheimer’s disease are associated with the functional impairment of the choroid plexus. One of the major causes underlying congenital hydrocephalus, an abnormal buildup of CSF in the brain ventricles that affects 1 out of every 1,000 newborns, is the abnormality of the choroid plexus. Moreover, it is recently found that, rather than neurons or glia in the central nervous system, it is SARS-CoV-2 infecting the choroid plexus that causes damaging of the epithelial blood-CSF barrier and leads to neurological complications in COVID-19 patients. Despite the pivotal role of the choroid plexus in brain homeostasis and development, how blood-CSF barrier function is regulated at the choroid plexus remains largely unknown.
Study shows that SOX9 in the choroid plexus is essential to prevent leakage of undesired molecules into the cerebrospinal fluid
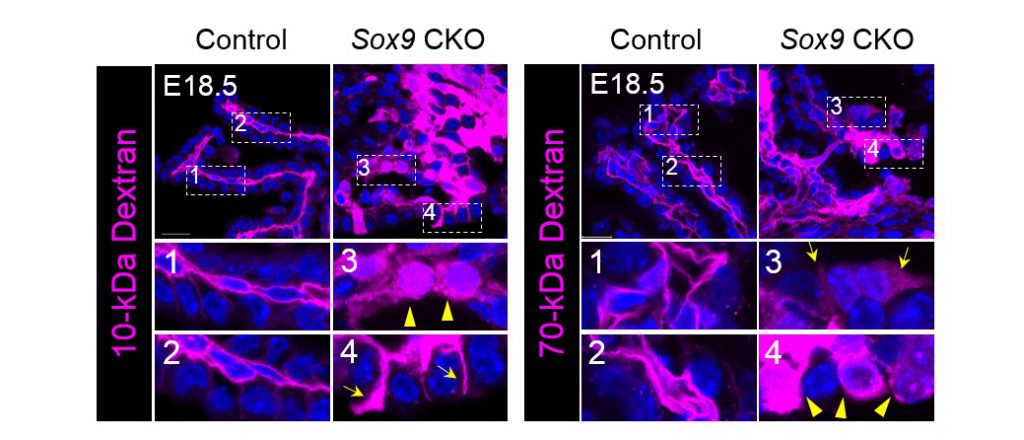
A research team led by Professor KWAN Kin Ming, Associate Professor from the School of Life Science at CUHK, has been dedicated in investigating the genetic regulation of neuronal cell development in the cerebellum. In previous experiments, they have observed the development of hydrocephalus in laboratory mice with genetic deletion of SOX9 from the choroid plexus epithelium, and this drew their attention. After further investigation, it was found that the loss of SOX9 function led to an abnormal increase in hyperpermeability of the blood-CSF barrier.
In the normal case, the choroid plexus restrains molecules in the blood stream from entering the CSF freely; the loss of SOX9 function resulted in the entry of fluorescent tracers into the brain tissue by passing through the choroid plexus into the CSF. In the absence of SOX9 function, there was a dramatic increase in the CSF protein level with an abnormal deposition of blood-borne proteins in the CSF of mutant mice, which is also a common phenomenon seen in patients with hydrocephalus. Such a change of CSF composition significantly affected proper brain development in mice.
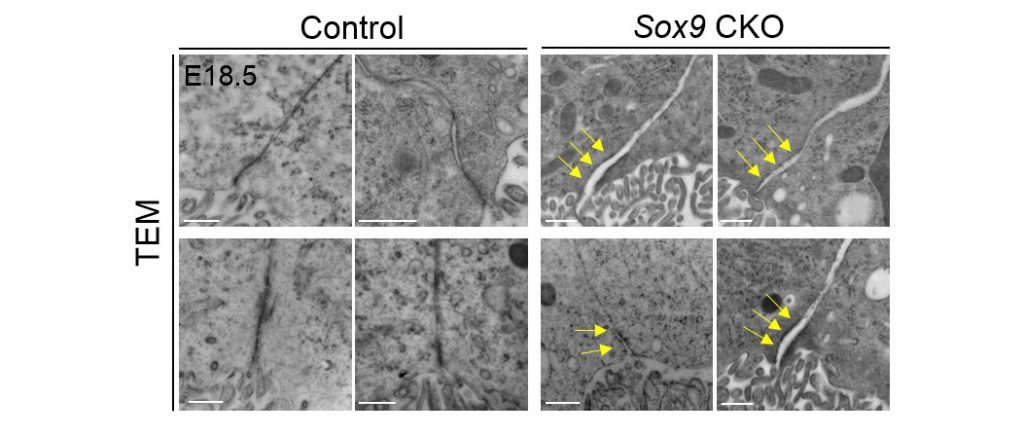
Through RNA sequencing, the team found that SOX9 is required for the synthesis of collagen IX at the choroid plexus epithelium. Using a temporal in utero gene knockdown approach, the team demonstrated that mice lacking collagen IX showed close resemblance to blood-CSF barrier impairment as seen in SOX9 mutants. Deficiency of collagen IX markedly increased the vulnerability of the basement membrane and, consequently, perturbed the polarised microtubule dynamics required for the maintenance of epithelial apicobasal polarity as well as the tight junction structures. These tight junctions, found between adjacent epithelial cells, are critical for restricting unauthorized passage of molecules across the choroid plexus.
Professor Kwan explained, “Recent advances in the field suggested that an abnormally permeable blood-CSF barrier is associated with neurodevelopmental disorders, for instance, congenital hydrocephalus and autism spectrum disorders. To understand how to prevent breaching of the barrier, or how to repair the permeable barrier, we must learn about the regulatory mechanisms behind the function of the blood-CSF barrier. Therapeutic strategies that aim at intervening in the function of blood-CSF barrier or modifying CSF constituents represent promising approaches to treating neurodevelopmental and neurological disorders. Based on what we have learnt in this research, we are now attempting to harness the choroid plexus function to alleviate CSF-related neurological disorders.”
This study was performed in collaboration with Professor Jiang Liwen, Professor Ngai Sai Ming and Professor Hui Ho Lam Jerome from the School of Life Sciences at CUHK. This project was supported by the General Research Fund, Collaborative Research Fund and Areas of Excellence (AoE) Scheme of the Hong Kong Research Grants Council, as well as the Centre for Cell and Developmental Biology and State Key Laboratory of Agrobiotechnology of CUHK.
The full text of the research paper can be found: https://www.pnas.org/content/118/6/e2009568118.
Brief biography of Prof. KWAN Kin Ming
Professor KWAN Kin Ming is Associate Dean (Education), Associate Professor and Director of the Natural Sciences Programme in the Faculty of Science at CUHK. He received undergraduate and doctoral training at The University of Hong Kong, and postdoctoral training at The University of Texas MD Anderson Cancer Center (US). Since joining CUHK in 2006, Professor Kwan has been investigating the genetic regulation of neural system development and its relationship to stem cell renewal and neuronal cell functioning, and was the recipient of the CUHK Young Researcher Award in 2008, and the CUHK Science Faculty Teaching Award in 2009 and 2013.

The research team from the School of Life Sciences at CUHK discovers that SOX9 protein is an essential regulatory factor of choroid plexus function that ensures the correct composition of cerebrospinal fluid (CSF). This is for the first time scientists uncover the regulatory mechanisms behind the permeability of blood-CSF barrier. The research team is led by Professor Kin-ming Kwan (third from the left), in collaboration with Professors Liwen Jiang (first from the left), Jerome Ho Lam Hui (second from the right) and Sai Ming Ngai (first from the right).
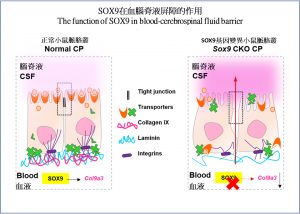
Under normal situation (image on the left), the closely packed choroid plexus restrains molecules in the blood stream from entering the CSF freely; the loss of SOX9 function (image on the right) resulted in the loss of collagen IX, eventually losing its protection against the entry of unwanted or harmful substance to the brain passing into the CSF.