The Puzzle of Pain: A CUHK Scholar's Nuanced Understanding of the Nervous System

Prof. Helen Wise
School of Biomedical Sciences
It is pain that drives Prof. Helen Wise, Professor of CUHK's School of Biomedical Sciences. Her investigation of cell signaling pathways has led to a nuanced understanding of how pain is transmitted in the body. It's a breakthrough that can lead to better drugs and treatment of chronic pain, such as crippling back pain, persistent migraines or injury-induced nerve damage. It could also improve treatment of trauma such as spine damage or heart attack.
All of us feel pain in our lives, and in a healthy human, it is a useful albeit unpleasant experience. We touch something hot, and recoil to prevent further damage. We are pinched by something, and try to remove the pressure.
It is when something goes wrong with the nervous system that the way we experience pain is transformed. What was a temporary response to warn us of a threat becomes chronic pain, and the threat in and of itself. Professor Wise has analyzed why that is, and what makes that happen.
"Everyone has back pain at some point," Professor Wise notes. But some people, thanks to nerve damage, are unable to walk, stand up, even get out of bed. She hopes her work can ease that kind of pain and allow people to function again. "It means you can help someone to have a better life, which is what a pharmacologist wants, really."
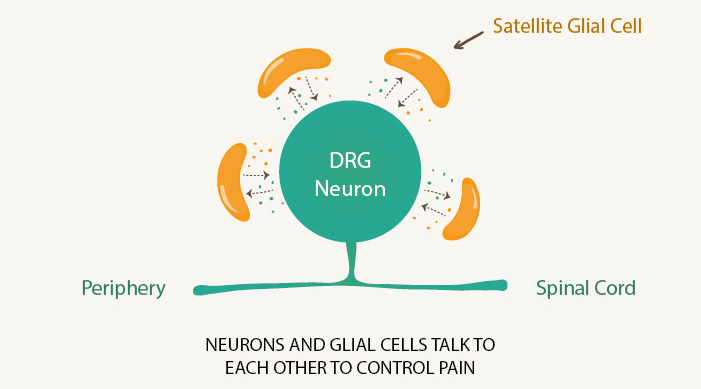
Professor Wise has focused on the relationship between neurons – our nerve cells – and the glial cells that surround them. In the past, scientists only thought of glial cells as support cells, that simply helped the neuron they surround to remain alive. They were seen as a way of removing dangerous material from the neuron and supplying it with elements to keep it healthy.
Professor Wise's work has discovered that glial cells don't just function as servants to the neurons but are in fact active players, helping to send messages from one cell to the other. "Glials and neurons talk," Professor Wise explains, and glial cells communicate with other glial cells.
Neurons fire neurotransmitters, messages sent to other neurons that can help shoot pain through our body. But Professor Wise has discovered that they also help to suppress glial cells, which fire off messages of their own.
The glial cells may, for instance, detect infection or damage and create an inflammatory response. They can make us sleepy so we get much-needed bed rest, or tell us to react to a cut finger. It's a useful acute response that can make our body kill off an infection or avert greater danger.
In the longer term, the neurons suppress the glial cells, keeping them in check. When neuron cells are dying or damaged, and unable to function as normal – such as in damage to the spinal cord – the glial cells run wild. The pain becomes unchecked.
Professor Wise began her research career in the private sector, at the pharmaceutical company Glaxo. "I never thought I would be an academic," she recalls, but got disillusioned when drug companies moved away from creating drugs to treat specific illnesses, and started screening for compounds where the therapeutic goal was remote. "You just became target-orientated," she says.
She found that her corporate background, where she worked on drugs that would affect many parts of the body, stood her in good stead. "I had studied virtually every system students need to know about: cardiovascular, digestive, neural. Most scientists just focus on one area."
That understanding also put her on the track in her study of pain transmission. The traditional scientific path is to study glial cells in isolation, and neurons in isolation. That involves separating them from each other, testing their function, growing them independently for several weeks and testing them again.
"These cells are now so far removed from the normal body that the conclusions aren't valid," Professor Wise says. "They tell you what a cell is capable of, but they don't tell you if that's what the cell does normally."
She has combined work in neurobiology, which focuses on the nervous system, and immunology, which looks at the body's self-defense systems. Like the study of glial cells and neurons, the two disciplines are typically tackled independently, but they cross over in the functioning of our bodies.
The interaction is complex. For instance, antibiotic drugs unexpectedly proved to be effective in treating nerve pain. Agents that are designed to fight infection also curb the activity of glial cells.
Professor Wise's understanding of the glial-neuron interaction also helps with treatment of pain through morphine, which works as a temporary pain reliever but in the long run can actually increase pain.
While morphine's short-term use numbs neurons, reducing pain transmission, Professor Wise has established that morphine can also stimulate what's known as TLR4, or the Toll-like receptor-4, which causes cells in the spinal cord to become inflamed. The morphine ceases to suppress pain and in fact causes it.
It's that complex interaction that fascinates Professor Wise. "Pain isn't the same thing for everybody," she says. "The stimulus that causes pain might be different but seems to have the same description. I'm helping to understand what might be going on."
By Alex Frew McMillan
This article was originally published on CUHK Homepage in Apr 2014.

