Home > Resources > Secondary School Corner > Resource Book for Sixth-Form Practical Chemistry (Photos - Experiment 9)

Resource Book for Sixth-Form Practical Chemistry
Experiment 9
Isolation of the Essential Oils from Common Spices and Spectroscopic Analysis of Their Major Constituents
 |
Whole cloves |
 |
Cinnamon barks |
 |
Cinnamon barks ground into small pieces |
 |
Cinnamon barks soaked in ethyl ethanoate |
 |
Heating the cinnamon barks in refluxing ethyl ethanoate (solid-liquid extraction) |
 |
|
 |
After heating under reflux for 20 minutes, the essential oil of the cinnamon barks was extracted into ethyl ethanoate |
 |
The decanted ethyl ethanoate solution containing the essential oil of cinnamon |
 |
Drying the organic solution by anhydrous magnesium sulphate |
 |
Removing the magnesium sulphate powder and other solid residue by simple filtration |
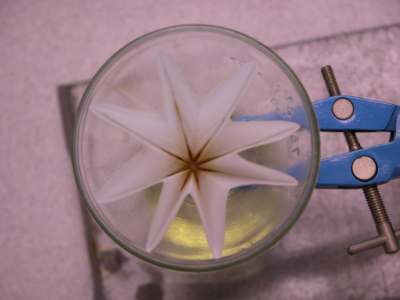 |
The solid residue remained in the filter paper |
 |
The filtrate: the dried solution of the essential oil of cinnamon in ethyl ethanoate |
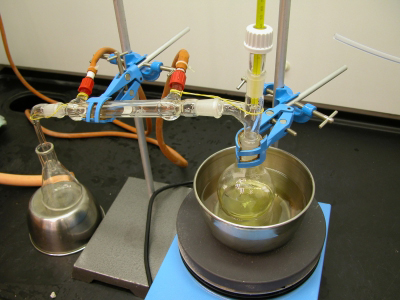 |
Removing the organic solvent (ethyl ethanoate) from the solution |
 |
The essential oil of cinnamon collected as yellowish oily residue (the white/gray solids are anti-bumping granules) |
2004 Department of Chemistry, The Chinese University of Hong Kong


